Fluid Biomarkers in Individuals at Risk for Genetic Prion Disease up to Disease Conversion
- PMID: 38896810
- PMCID: PMC11226308
- DOI: 10.1212/WNL.0000000000209506
Fluid Biomarkers in Individuals at Risk for Genetic Prion Disease up to Disease Conversion
Abstract
Objectives: To longitudinally characterize disease-relevant CSF and plasma biomarkers in individuals at risk for genetic prion disease up to disease conversion.
Methods: This single-center longitudinal cohort study has followed known carriers of PRNP pathogenic variants at risk for prion disease, individuals with a close relative who died of genetic prion disease but who have not undergone predictive genetic testing, and controls. All participants were asymptomatic at first visit and returned roughly annually. We determined PRNP genotypes, measured NfL and GFAP in plasma, and RT-QuIC, total PrP, NfL, T-tau, and beta-synuclein in CSF.
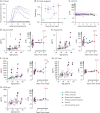
Results: Among 41 carriers and 21 controls enrolled, 28 (68%) and 15 (71%) were female, and mean ages were 47.5 and 46.1. At baseline, all individuals were asymptomatic. We observed RT-QuIC seeding activity in the CSF of 3 asymptomatic E200K carriers who subsequently converted to symptomatic and died of prion disease. 1 P102L carrier remained RT-QuIC negative through symptom conversion. No other individuals developed symptoms. The prodromal window from detection of RT-QuIC positivity to disease onset was 1 year long in an E200K individual homozygous (V/V) at PRNP codon 129 and 2.5 and 3.1 years in 2 codon 129 heterozygotes (M/V). Changes in neurodegenerative and neuroinflammatory markers were variably observed prior to onset, with increases observed for plasma NfL in 4/4 converters, and plasma GFAP, CSF NfL, CSF T-tau, and CSF beta-synuclein each in 2/4 converters, although values relative to age and fold changes relative to individual baseline were not remarkable for any of these markers. CSF PrP was longitudinally stable with mean coefficient of variation 9.0% across all individuals over up to 6 years, including data from converting individuals at RT-QuIC-positive timepoints.
Discussion: CSF prion seeding activity may represent the earliest detectable prodromal sign in E200K carriers. Neuronal damage and neuroinflammation markers show limited sensitivity in the prodromal phase. CSF PrP levels remain stable even in the presence of RT-QuIC seeding activity.
Clinical trials registration: ClinicalTrials.gov NCT05124392 posted 2017-12-01, updated 2023-01-27.
Conflict of interest statement
S.M. Vallabh has received speaking fees from Ultragenyx, Illumina, Biogen, Eli Lilly, consulting fees from Invitae and Alnylam, and research support from Ionis, Gate, Sangamo. E.V. Minikel has received speaking fees from Eli Lilly, consulting fees from Deerfield and Alnylam, and research support from Ionis, Gate, Sangamo, Eli Lilly. S.E. Arnold has received speaking fees from Abbvie, Biogen, EIP Pharma, Roche, and Sironax, consulting fees from Athira, Biogen, Cassava, Cognito, Cortexyme, Sironax, and vTv, and research support from Abbvie, Amylyx, EIP Pharma, and Merck. The other authors report no relevant disclosures. Go to
Figures
Update of
-
Biomarker changes preceding symptom onset in genetic prion disease.medRxiv [Preprint]. 2023 Dec 18:2023.12.18.23300042. doi: 10.1101/2023.12.18.23300042. medRxiv. 2023. Update in: Neurology. 2024 Jul 23;103(2):e209506. doi: 10.1212/WNL.0000000000209506. PMID: 38196583 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
