Sodium Manganese Ferrite Water Splitting Cycle: Unravelling the Effect of Solid-Liquid Interfaces in Molten Alkali Carbonates
- PMID: 38896815
- PMCID: PMC11231967
- DOI: 10.1021/acsami.4c00549
Sodium Manganese Ferrite Water Splitting Cycle: Unravelling the Effect of Solid-Liquid Interfaces in Molten Alkali Carbonates
Abstract
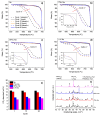
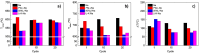
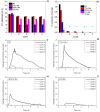
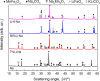
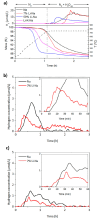
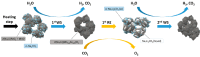
In this work, the Na2CO3 of the sodium manganese ferrite thermochemical cycle was substituted by different eutectic or eutectoid alkali carbonate mixtures. Substituting Na2CO3 with the eutectoid (Li0.07Na0.93)2CO3 mixture resulted in faster hydrogen production after the first cycle, shifting the hydrogen production maximum toward shorter reaction times. Thermodynamic calculations and in situ optical microscopy attributed this fact to the partial melting of the eutectoid carbonate, which helps the diffusion of the ions. Unfortunately, all the mixtures exhibit a significant loss of reversibility in terms of hydrogen production upon cycling. Among them, the nonsubstituted Na mixture exhibits the highest reversibility in terms of hydrogen production followed by the 7%Li-Na mixture, while the 50%Li-Na and Li-K-Na mixtures do not produce any hydrogen after the first cycle. The loss of reversibility is attributed to both the formation of undesired phases and sintering, the latter being more pronounced in the eutectic and eutectoid alkali carbonate mixtures, where the melting of the carbonate is predicted by thermodynamics.
Keywords: atomic substitution; carbonation; decarbonation; hydrogen production; sodium manganese ferrite cycle; thermochemical water splitting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Griffiths S.; Sovacool B. K.; Kim J.; Bazilian M.; Uratani J. M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Research and Social Science. 2021, 80, 102208.10.1016/j.erss.2021.102208. - DOI
-
- Lin R. H.; Zhao Y. Y.; Wu B. D. Toward a Hydrogen Society: Hydrogen and Smart Grid Integration. Int. J. Hydrogen Energy 2020, 45 (39), 20164.10.1016/j.ijhydene.2020.01.047. - DOI
LinkOut - more resources
Full Text Sources

