Development of a PCSK9-targeted nanoparticle vaccine to effectively decrease the hypercholesterolemia
- PMID: 38897173
- PMCID: PMC11228807
- DOI: 10.1016/j.xcrm.2024.101614
Development of a PCSK9-targeted nanoparticle vaccine to effectively decrease the hypercholesterolemia
Abstract
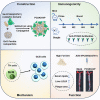
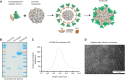
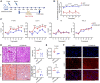
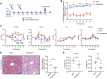
Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to the low-density lipoprotein receptor (LDLR) and mediates its internalization and degradation, resulting in an increase in LDL cholesterol levels. Recently, PCSK9 emerged as a therapeutic target for hypercholesterolemia and atherosclerosis. In this study, we develop a PCSK9 nanoparticle (NP) vaccine by covalently conjugating the catalytic domain (aa 153-aa 454, D374Y) of PCSK9 to self-assembled 24-mer ferritin NPs. We demonstrate that the PCSK9 NP vaccine effectively induces interfering antibodies against PCSK9 and reduces serum lipids levels in both a high-fat diet-induced hypercholesterolemia model and an adeno-associated virus-hPCSK9D374Y-induced hypercholesterolemia model. Additionally, the vaccine significantly reduces plaque lesion areas in the aorta and macrophages infiltration in an atherosclerosis mouse model. Furthermore, we discover that the vaccine's efficacy relied on T follicular help cells and LDLR. Overall, these findings suggest that the PCSK9 NP vaccine holds promise as an effective treatment for hypercholesterolemia and atherosclerosis.
Keywords: LDLR; PCSK9; T follicular help cells; hypercholesterolemia; nanoparticle vaccine.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors have no conflict of interest.
Figures




References
-
- Tsimikas S., Fazio S., Ferdinand K.C., Ginsberg H.N., Koschinsky M.L., Marcovina S.M., Moriarty P.M., Rader D.J., Remaley A.T., Reyes-Soffer G., et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018;71:177–192. doi: 10.1016/j.jacc.2017.11.014. - DOI - PMC - PubMed
-
- Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. - DOI - PMC - PubMed
-
- Crisby M., Nordin-Fredriksson G., Shah P.K., Yano J., Zhu J., Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. - DOI - PubMed
-
- Nicholls S.J., Ballantyne C.M., Barter P.J., Chapman M.J., Erbel R.M., Libby P., Raichlen J.S., Uno K., Borgman M., Wolski K., Nissen S.E. Effect of Two Intensive Statin Regimens on Progression of Coronary Disease. N. Engl. J. Med. 2011;365:2078–2087. doi: 10.1097/01.sa.0000414260.89578.71. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

