Therapeutic potential of human microglia transplantation in a chimeric model of CSF1R-related leukoencephalopathy
- PMID: 38897209
- PMCID: PMC12357648
- DOI: 10.1016/j.neuron.2024.05.023
Therapeutic potential of human microglia transplantation in a chimeric model of CSF1R-related leukoencephalopathy
Abstract
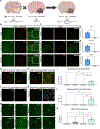
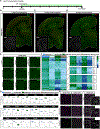
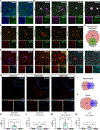
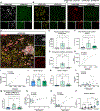
Microglia replacement strategies are increasingly being considered for the treatment of primary microgliopathies like adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). However, available mouse models fail to recapitulate the diverse neuropathologies and reduced microglia numbers observed in patients. In this study, we generated a xenotolerant mouse model lacking the fms-intronic regulatory element (FIRE) enhancer within Csf1r, which develops nearly all the hallmark pathologies associated with ALSP. Remarkably, transplantation of human induced pluripotent stem cell (iPSC)-derived microglial (iMG) progenitors restores a homeostatic microglial signature and prevents the development of axonal spheroids, white matter abnormalities, reactive astrocytosis, and brain calcifications. Furthermore, transplantation of CRISPR-corrected ALSP-patient-derived iMG reverses pre-existing spheroids, astrogliosis, and calcification pathologies. Together with the accompanying study by Munro and colleagues, our results demonstrate the utility of FIRE mice to model ALSP and provide compelling evidence that iMG transplantation could offer a promising new therapeutic strategy for ALSP and perhaps other microglia-associated neurological disorders.
Keywords: ALSP; CRISPR correction; CSF1R; FIRE; axonal spheroids; chimera; humanized; leukoencephalopathy; microglia.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.P.C., J.H., R.C.S., S.G. H.D., and M.B.-J. are co-inventors on patent applications filed by the University of California Regents (US 63/169,578) related to genetic modification of cells to confer resistance to CSF1R antagonists and (US 63/388,766) related to transplantation of stem-cell-derived microglia to treat leukodystrophies. M.B.-J. is a co-inventor of patent application WO/2018/160496, related to the differentiation of human pluripotent stem cells into microglia. M.B.-J., S.G., and R.C.S. are co-founders of NovoGlia Inc. Z.K.W. serves as principal investigator (PI) or co-PI on Biohaven Pharmaceuticals, Inc. (BHV4157-206) and Vigil Neuroscience, Inc. (VGL101-01.002, VGL101-01.201, PET tracer development protocol, Csf1r biomarker and repository project, and ultra-high-field MRI in the diagnosis and management of CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia) projects/grants. He serves as co-PI of the Mayo Clinic APDA Center for Advanced Research; as an external advisory board member for Vigil Neuroscience, Inc.; and as a consultant on neurodegenerative medical research for Eli Lilli & Company.
Figures



References
-
- Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, et al. (2011). Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 44, 200–205. 10.1038/ng.1027. - DOI - PMC - PubMed
-
- Konno T, Yoshida K, Mizuno T, Kawarai T, Tada M, Nozaki H, Ikeda SI, Nishizawa M, Onodera O, Wszolek ZK, and Ikeuchi T (2017). Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur J Neurol 24, 37–45. 10.1111/ene.13125. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

