Dissecting the abilities of murine Siglecs to interact with gangliosides
- PMID: 38897567
- PMCID: PMC11294694
- DOI: 10.1016/j.jbc.2024.107482
Dissecting the abilities of murine Siglecs to interact with gangliosides
Abstract
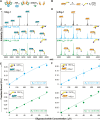
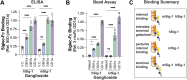
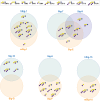
Siglecs are cell surface receptors whose functions are tied to the binding of their sialoglycan ligands. Recently, we developed an optimized liposome formulation and used it to investigate the binding of human Siglecs (hSiglec) against a panel of gangliosides. Animal models, more specifically murine models, are used to understand human biology; however, species-specific differences can complicate the interpretation of the results. Herein, we used our optimized liposome formulation to dissect the interactions between murine Siglecs (mSiglecs) and gangliosides to assess the appropriateness of mSiglecs as a proxy to better understand the biological roles of hSiglec-ganglioside interactions. Using our optimized liposome formulation, we found that ganglioside binding is generally conserved between mice and humans with mSiglec-1, -E, -F, and -15 binding multiple gangliosides like their human counterparts. However, in contrast to the hSiglecs, we observed little to no binding between the mSiglecs and ganglioside GM1a. Detailed analysis of mSiglec-1 interacting with GM1a and its structural isomer, GM1b, suggests that mSiglec-1 preferentially binds α2-3-linked sialic acids presented from the terminal galactose residue. The ability of mSiglecs to interact or not interact with gangliosides, particularly GM1a, has implications for using mice to study neurodegenerative diseases, infections, and cancer, where interactions between Siglecs and glycolipids have been proposed to modulate these human diseases.
Keywords: Siglec; flow cytometry; ganglioside; glycolipid; liposome; mass spectrometry; mouse.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

