Nab-paclitaxel, cisplatin, and capecitabine versus cisplatin and gemcitabine as first line chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma: randomised phase 3 clinical trial
- PMID: 38897625
- PMCID: PMC11190944
- DOI: 10.1136/bmj-2023-077890
Nab-paclitaxel, cisplatin, and capecitabine versus cisplatin and gemcitabine as first line chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma: randomised phase 3 clinical trial
Erratum in
-
Nab-paclitaxel, cisplatin, and capecitabine versus cisplatin and gemcitabine as first line chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma: randomised phase 3 clinical trial.BMJ. 2024 Jun 21;385:q1381. doi: 10.1136/bmj.q1381. BMJ. 2024. PMID: 38906541 Free PMC article. No abstract available.
Abstract
Objective: To compare the effectiveness and safety of nab-paclitaxel, cisplatin, and capecitabine (nab-TPC) with gemcitabine and cisplatin as an alternative first line treatment option for recurrent or metastatic nasopharyngeal carcinoma.
Design: Phase 3, open label, multicentre, randomised trial.
Setting: Four hospitals located in China between September 2019 and August 2022.
Participants: Adults (≥18 years) with recurrent or metastatic nasopharyngeal carcinoma.
Interventions: Patients were randomised in a 1:1 ratio to treatment with either nab-paclitaxel (200 g/m2 on day 1), cisplatin (60 mg/m2 on day 1), and capecitabine (1000 mg/m2 twice on days 1-14) or gemcitabine (1 g/m2 on days 1 and 8) and cisplatin (80 mg/m2 on day 1).
Main outcome measures: Progression-free survival was evaluated by the independent review committee as the primary endpoint in the intention-to-treat population.
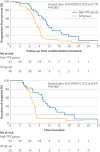
Results: The median follow-up was 15.8 months in the prespecified interim analysis (31 October 2022). As assessed by the independent review committee, the median progression-free survival was 11.3 (95% confidence interval 9.7 to 12.9) months in the nab-TPC cohort compared with 7.7 (6.5 to 9.0) months in the gemcitabine and cisplatin cohort. The hazard ratio was 0.43 (95% confidence interval 0.25 to 0.73; P=0.002). The objective response rate in the nab-TPC cohort was 83% (34/41) versus 63% (25/40) in the gemcitabine and cisplatin cohort (P=0.05), and the duration of response was 10.8 months in the nab-TPC cohort compared with 6.9 months in the gemcitabine and cisplatin cohort (P=0.009). Treatment related grade 3 or 4 adverse events, including leukopenia (4/41 (10%) v 13/40 (33%); P=0.02), neutropenia (6/41 (15%) v 16/40 (40%); P=0.01), and anaemia (1/41 (2%) v 8/40 (20%); P=0.01), were higher in the gemcitabine and cisplatin cohort than in the nab-TPC cohort. No deaths related to treatment occurred in either treatment group. Survival and long term toxicity are still being evaluated with longer follow-up.
Conclusion: The nab-TPC regimen showed a superior antitumoural efficacy and favourable safety profile compared with gemcitabine and cisplatin for recurrent or metastatic nasopharyngeal carcinoma. Nab-TPC should be considered the standard first line treatment for recurrent or metastatic nasopharyngeal carcinoma. Longer follow-up is needed to confirm the benefits for overall survival.
Trial registration: Chinese Clinical Trial Registry ChiCTR1900027112.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Guangdong Basic and Applied Basic Research Foundation, National Natural Science Foundation of China, CPSF, and Postdoctoral Fellowship Program of CPSF for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Sun Y, Li WF, Chen NY, et al. . Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. 10.1016/S1470-2045(16)30410-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
