UK neonatal stoma practice: a population study
- PMID: 38897635
- PMCID: PMC11671890
- DOI: 10.1136/archdischild-2024-327020
UK neonatal stoma practice: a population study
Abstract
Objective: The optimal time for neonatal stoma closure is unclear and there have been calls for a trial to compare early and late surgery. The feasibility of such a trial will depend on the population of eligible infants and acceptability to families and health professionals. In this study, we aimed to determine current UK practice and characteristics of those undergoing stoma surgery.
Design: A retrospective cohort study of neonates who had undergone stoma surgery (excluding anorectal malformations and Hirschsprung's disease) using three national databases: the National Neonatal Research Database (NNRD, 2012-2019), British Association of Paediatric Surgeons Congenital Anomalies Surveillance System (BAPS-CASS, 2013-2014) and Hospital Episode Statistics-Admitted Patient Care (HES-APC, 2011-2018).
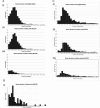
Results: 1830 eligible neonates were identified from NNRD, 163 from BAPS-CASS, 2477 from HES-APC. Median (IQR) duration of stoma in days was 57 (36-80) in NNRD, 63 (41-130) in BAPS-CASS and 78 (55-122) for neonates identified from HES-APC. At the time of closure, there were low rates of invasive ventilation (13%), inotrope use (5%) and recent steroids use (4%). Infants who underwent earlier closure (<9 weeks) were less preterm (median 28 weeks vs 25 weeks), have higher birth weight (median 986 g vs 764 g) and more likely to have stoma complications (29% vs 5%).
Conclusion: There are sufficient UK neonates undergoing stoma formation for a trial. Stoma closure is performed at around 2 months, with clinical stability, gestation, weight and stoma complications appearing to influence timing. The variation in practice we document indicates there is opportunity to optimise practice through a trial.
Keywords: Gastroenterology; Intensive Care Units, Neonatal; Neonatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
