A Light-Responsive Neural Circuit Suppresses Feeding
- PMID: 38897723
- PMCID: PMC11270527
- DOI: 10.1523/JNEUROSCI.2192-23.2024
A Light-Responsive Neural Circuit Suppresses Feeding
Abstract
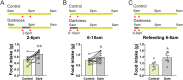
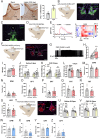
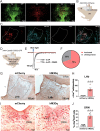
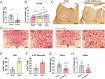
Light plays an essential role in a variety of physiological processes, including vision, mood, and glucose homeostasis. However, the intricate relationship between light and an animal's feeding behavior has remained elusive. Here, we found that light exposure suppresses food intake, whereas darkness amplifies it in male mice. Interestingly, this phenomenon extends its reach to diurnal male Nile grass rats and healthy humans. We further show that lateral habenula (LHb) neurons in mice respond to light exposure, which in turn activates 5-HT neurons in the dorsal Raphe nucleus (DRN). Activation of the LHb→5-HTDRN circuit in mice blunts darkness-induced hyperphagia, while inhibition of the circuit prevents light-induced anorexia. Together, we discovered a light-responsive neural circuit that relays the environmental light signals to regulate feeding behavior in mice.
Keywords: 5-HT; LHb; feeding; light.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
