Distinct Action Signals by Subregions in the Nucleus Accumbens during STOP-Change Performance
- PMID: 38897724
- PMCID: PMC11255435
- DOI: 10.1523/JNEUROSCI.0020-24.2024
Distinct Action Signals by Subregions in the Nucleus Accumbens during STOP-Change Performance
Abstract
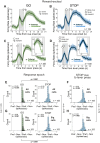
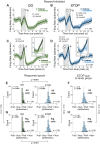
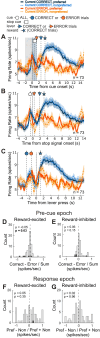
The nucleus accumbens (NAc) is thought to contribute to motivated behavior by signaling the value of reward-predicting cues and the delivery of anticipated reward. The NAc is subdivided into core and shell, with each region containing different populations of neurons that increase or decrease firing to rewarding events. While there are numerous theories of functions pertaining to these subregions and cell types, most are in the context of reward processing, with fewer considering that the NAc might serve functions related to action selection more generally. We recorded from single neurons in the NAc as rats of both sexes performed a STOP-change task that is commonly used to study motor control and impulsivity. In this task, rats respond quickly to a spatial cue on 80% of trials (GO) and must stop and redirect planned movement on 20% of trials (STOP). We found that the activity of reward-excited neurons signaled accurate response direction on GO, but not STOP, trials and that these neurons exhibited higher precue firing after correct trials. In contrast, reward-inhibited neurons significantly represented response direction on STOP trials at the time of the instrumental response. Finally, the proportion of reward-excited to reward-inhibited neurons and the strength of precue firing decreased as the electrode traversed the NAc. We conclude that reward-excited cells (more common in core) promote proactive action selection, while reward-inhibited cells (more common in shell) contribute to accurate responding on STOP trials that require reactive suppression and redirection of behavior.
Keywords: action selection; impulsivity; inhibition; nucleus accumbens; rat; single unit; stop-signal.
Copyright © 2024 Ashton et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Barto AG (1995) Adaptive critics and the basal ganglia. In: Models of information processing in the basal ganglia (Houk JC, Davis JL, Beiser DG, eds), pp 215–232. Cambridge, MA: MIT Press.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
