Impact of SARS-CoV-2 infection during pregnancy on the placenta and fetus
- PMID: 38897829
- PMCID: PMC11288977
- DOI: 10.1016/j.semperi.2024.151919
Impact of SARS-CoV-2 infection during pregnancy on the placenta and fetus
Abstract
Pregnant people and their fetuses are vulnerable to adverse health outcomes from coronavirus 2019 disease (COVID-19) due to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has been associated with higher rates of maternal mortality, preterm birth, and stillbirth. While SARS-CoV-2 infection of the placenta and vertical transmission is rare, this may be due to the typically longer time interval between maternal infection and testing of the placenta and neonate. Placental injury is evident in cases of SARS-CoV-2-associated stillbirth with massive perivillous fibrin deposition, chronic histiocytic intervillositis, and trophoblast necrosis. Maternal COVID-19 can also polarize fetal immunity, which may have long-term effects on neurodevelopment. Although the COVID-19 pandemic continues to evolve, the impact of emerging SARS-CoV-2 variants on placental and perinatal injury/mortality remains concerning for maternal and perinatal health. Here, we highlight the impact of COVID-19 on the placenta and fetus and remaining knowledge gaps.
Keywords: COVID-19; Coronavirus; Fetus; Immune response; Maternal mortality; Placenta; Pregnancy; Preterm birth; SARS-CoV-2; Stillbirth; Vertical transmission.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement The authors report no conflict of interest.
Figures

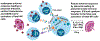
Similar articles
-
Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis.Arch Pathol Lab Med. 2021 Nov 1;145(11):1341-1349. doi: 10.5858/arpa.2021-0246-SA. Arch Pathol Lab Med. 2021. PMID: 34338723
-
Chronic Histiocytic Intervillositis With Trophoblast Necrosis Is a Risk Factor Associated With Placental Infection From Coronavirus Disease 2019 (COVID-19) and Intrauterine Maternal-Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Live-Born and Stillborn Infants.Arch Pathol Lab Med. 2021 May 1;145(5):517-528. doi: 10.5858/arpa.2020-0771-SA. Arch Pathol Lab Med. 2021. PMID: 33393592
-
Placental lesions and SARS-Cov-2 infection: Diffuse placenta damage associated to poor fetal outcome.Placenta. 2021 Sep 1;112:97-104. doi: 10.1016/j.placenta.2021.07.288. Epub 2021 Jul 15. Placenta. 2021. PMID: 34329973 Free PMC article.
-
Placental Pathology of COVID-19 with and without Fetal and Neonatal Infection: Trophoblast Necrosis and Chronic Histiocytic Intervillositis as Risk Factors for Transplacental Transmission of SARS-CoV-2.Viruses. 2020 Nov 15;12(11):1308. doi: 10.3390/v12111308. Viruses. 2020. PMID: 33203131 Free PMC article. Review.
-
Coronavirus Diseases in Pregnant Women, the Placenta, Fetus, and Neonate.Adv Exp Med Biol. 2021;1318:223-241. doi: 10.1007/978-3-030-63761-3_14. Adv Exp Med Biol. 2021. PMID: 33973182 Review.
Cited by
-
Oropouche Virus (OROV) in Pregnancy: An Emerging Cause of Placental and Fetal Infection Associated with Stillbirth and Microcephaly following Vertical Transmission.Viruses. 2024 Sep 9;16(9):1435. doi: 10.3390/v16091435. Viruses. 2024. PMID: 39339911 Free PMC article. Review.
-
Risk of Transmission of COVID-19 from the Mother to the Foetus: A Systematic Review.J Mother Child. 2024 Nov 20;28(1):94-101. doi: 10.34763/jmotherandchild.20242801.d-24-00032. eCollection 2024 Feb 1. J Mother Child. 2024. PMID: 39561303 Free PMC article.
-
Risk of neonatal SARS-CoV-2 infection: a retrospective cohort study based on infected mothers with gestational diabetes mellitus.Front Endocrinol (Lausanne). 2025 Jan 30;16:1483962. doi: 10.3389/fendo.2025.1483962. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 39950026 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

