CSTF3 contributes to platinum resistance in ovarian cancer through alternative polyadenylation of lncRNA NEAT1 and generating the short isoform NEAT1_1
- PMID: 38898019
- PMCID: PMC11187223
- DOI: 10.1038/s41419-024-06816-1
CSTF3 contributes to platinum resistance in ovarian cancer through alternative polyadenylation of lncRNA NEAT1 and generating the short isoform NEAT1_1
Abstract
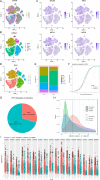
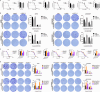
Platinum-based chemotherapy is the standard postoperative adjuvant treatment for ovarian cancer (OC). Despite the initial response to chemotherapy, 85% of advanced OC patients will have recurrent disease. Relapsed disease and platinum resistance are the major causes of death in OC patients. In this study, we compared the global regulation of alternative polyadenylation (APA) in platinum-resistant and platinum-sensitive tissues of OC patients by analyzing a set of single-cell RNA sequencing (scRNA-seq) data from public databases and found that platinum-resistant patients exhibited global 3' untranslated region (UTR) shortening due to the different usage of polyadenylation sites (PASs). The APA regulator CSTF3 was the most significantly upregulated gene in epithelial cells of platinum-resistant OC. CSTF3 knockdown increased the sensitivity of OC cells to platinum. The lncRNA NEAT1 has two isoforms, short (NEAT1_1) and long (NEAT1_2) transcript, because of the APA processing in 3'UTR. We found that CSTF3 knockdown reduced the usage of NEAT1 proximal PAS to lengthen the transcript and facilitate the expression of NEAT1_2. Downregulation of the expression of NEAT1 (NEAT1_1/_2), but not only NEAT1_2, also increased the sensitivity of OC cells to platinum. Overexpressed NEAT1_1 reversed the platinum resistance of OC cells after knocking down CSTF3 expression. Furthermore, downregulated expression of CSTF3 and NEAT1_1, rather than NEAT1_2, was positively correlated with inactivation of the PI3K/AKT/mTOR pathway in OC cells. Together, our findings revealed a novel mechanism of APA regulation in platinum-resistant OC. CSTF3 directly bound downstream of the NEAT1 proximal PAS to generate the short isoform NEAT1_1 and was conducive to platinum resistance, which provides a potential biomarker and therapeutic strategy for platinum-resistant OC patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
IGF2BP2 binding to CPSF6 facilitates m6A-mediated alternative polyadenylation of PUM2 and promotes malignant progression in ovarian cancer.Clin Transl Med. 2025 Jul;15(7):e70388. doi: 10.1002/ctm2.70388. Clin Transl Med. 2025. PMID: 40629911 Free PMC article.
-
Isoform balance of the long noncoding RNA NEAT1 is regulated by the RNA-binding protein QKI, governs the glioma transcriptome, and impacts cell migration.J Biol Chem. 2024 Aug;300(8):107595. doi: 10.1016/j.jbc.2024.107595. Epub 2024 Jul 18. J Biol Chem. 2024. PMID: 39032650 Free PMC article.
-
piR-1919609 Is an Ideal Potential Target for Reversing Platinum Resistance in Ovarian Cancer.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241249692. doi: 10.1177/15330338241249692. Technol Cancer Res Treat. 2024. PMID: 38706262 Free PMC article.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
Cited by
-
Immune evasion from macrophages by NEAT1-induced CD24 in liver cancer.Oncogene. 2025 Aug 12. doi: 10.1038/s41388-025-03537-3. Online ahead of print. Oncogene. 2025. PMID: 40796954
-
IGF2BP2 binding to CPSF6 facilitates m6A-mediated alternative polyadenylation of PUM2 and promotes malignant progression in ovarian cancer.Clin Transl Med. 2025 Jul;15(7):e70388. doi: 10.1002/ctm2.70388. Clin Transl Med. 2025. PMID: 40629911 Free PMC article.
-
IGF2BP3 recruits NUDT21 to regulate SPTBN1 alternative polyadenylation and drive ovarian cancer progression.Commun Biol. 2025 Apr 29;8(1):680. doi: 10.1038/s42003-025-08097-6. Commun Biol. 2025. PMID: 40301554 Free PMC article.
-
Identifying the ceRNA Regulatory Network in Early-Stage Acute Pancreatitis and Investigating the Therapeutic Potential of NEAT1 in Mouse Models.J Inflamm Res. 2024 Nov 2;17:8099-8115. doi: 10.2147/JIR.S490315. eCollection 2024. J Inflamm Res. 2024. PMID: 39507263 Free PMC article.
-
Latest Update on lncRNA in Epithelial Ovarian Cancer-A Scoping Review.Cells. 2025 Apr 7;14(7):555. doi: 10.3390/cells14070555. Cells. 2025. PMID: 40214508 Free PMC article.
References
-
- Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;37:2968–73. doi: 10.1200/JCO.18.02238. - DOI - PMC - PubMed
-
- Lee JM, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol. 2018;19:207–15. doi: 10.1016/S1470-2045(18)30009-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

