Palladium-catalyzed Suzuki-Miyaura cross-couplings of stable glycal boronates for robust synthesis of C-1 glycals
- PMID: 38898022
- PMCID: PMC11187158
- DOI: 10.1038/s41467-024-49547-9
Palladium-catalyzed Suzuki-Miyaura cross-couplings of stable glycal boronates for robust synthesis of C-1 glycals
Abstract
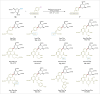
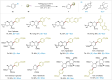
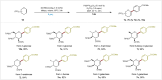
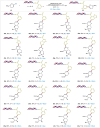
C-1 Glycals serve as pivotal intermediates in synthesizing diverse C-glycosyl compounds and natural products, necessitating the development of concise, efficient and user-friendly methods to obtain C-1 glycosides is essential. The Suzuki-Miyaura cross-coupling of glycal boronates is notable for its reliability and non-toxic nature, but glycal donor stability remains a challenge. Herein, we achieve a significant breakthrough by developing stable glycal boronates, effectively overcoming the stability issue in glycal-based Suzuki-Miyaura coupling. Leveraging the balanced reactivity and stability of our glycal boronates, we establish a robust palladium-catalyzed glycal-based Suzuki-Miyaura reaction, facilitating the formation of various C(sp2)-C(sp), C(sp2)-C(sp2), and C(sp2)-C(sp3) bonds under mild conditions. Notably, we expand upon this achievement by developing the DNA-compatible glycal-based cross-coupling reaction to synthesize various glycal-DNA conjugates. With its excellent reaction reactivity, stability, generality, and ease of handling, the method holds promise for widespread appication in the preparation of C-glycosyl compounds and natural products.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

