Broad-spectrum activity against mosquito-borne flaviviruses achieved by a targeted protein degradation mechanism
- PMID: 38898037
- PMCID: PMC11187112
- DOI: 10.1038/s41467-024-49161-9
Broad-spectrum activity against mosquito-borne flaviviruses achieved by a targeted protein degradation mechanism
Abstract
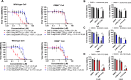
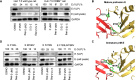
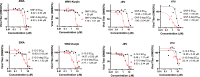
Viral genetic diversity presents significant challenges in developing antivirals with broad-spectrum activity and high barriers to resistance. Here we report development of proteolysis targeting chimeras (PROTACs) targeting the dengue virus envelope (E) protein through coupling of known E fusion inhibitors to ligands of the CRL4CRBN E3 ubiquitin ligase. The resulting small molecules block viral entry through inhibition of E-mediated membrane fusion and interfere with viral particle production by depleting intracellular E in infected Huh 7.5 cells. This activity is retained in the presence of point mutations previously shown to confer partial resistance to the parental inhibitors due to decreased inhibitor-binding. The E PROTACs also exhibit broadened spectrum of activity compared to the parental E inhibitors against a panel of mosquito-borne flaviviruses. These findings encourage further exploration of targeted protein degradation as a differentiated and potentially advantageous modality for development of broad-spectrum direct-acting antivirals.
© 2024. The Author(s).
Conflict of interest statement
N.S.G is a founder, science advisory board member (SAB) and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Shenandoah (board member), Larkspur (board member) and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline and Sanofi. E.S.F. is a founder, member of the scientific advisory board (SAB), and equity holder of Civetta Therapeutics, Lighthorse, Proximity Therapeutics, and Neomorph Inc (also board of directors), SAB member and equity holder in Avilar Therapeutics and Photys Therapeutics, and a consultant to Astellas, Sanofi, Novartis, Deerfield, Ajax and EcoR1 capital. The Fischer laboratory receives or has received research funding from Novartis, Deerfield, Ajax, Interline, and Astellas. K.A.D receives or has received consulting fees from Kronos Bio and Neomorph Inc. T.Z. is a scientific founder, equity holder, and consultant of Matchpoint, equity holder of Shenandoah. H.L. is currently an employee of Amgen. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

