Biosynthesis of GMGT lipids by a radical SAM enzyme associated with anaerobic archaea and oxygen-deficient environments
- PMID: 38898040
- PMCID: PMC11186832
- DOI: 10.1038/s41467-024-49650-x
Biosynthesis of GMGT lipids by a radical SAM enzyme associated with anaerobic archaea and oxygen-deficient environments
Abstract
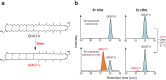
Archaea possess characteristic membrane-spanning lipids that are thought to contribute to the adaptation to extreme environments. However, the biosynthesis of these lipids is poorly understood. Here, we identify a radical S-adenosyl-L-methionine (SAM) enzyme that synthesizes glycerol monoalkyl glycerol tetraethers (GMGTs). The enzyme, which we name GMGT synthase (Gms), catalyzes the formation of a C(sp3)-C(sp3) linkage between the two isoprenoid chains of glycerol dialkyl glycerol tetraethers (GDGTs). This conclusion is supported by heterologous expression of gene gms from a GMGT-producing species in a methanogen, as well as demonstration of in vitro activity using purified Gms enzyme. Additionally, we show that genes encoding putative Gms homologs are present in obligate anaerobic archaea and in metagenomes obtained from oxygen-deficient environments, and appear to be absent in metagenomes from oxic settings.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Identification of two archaeal GDGT lipid-modifying proteins reveals diverse microbes capable of GMGT biosynthesis and modification.Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2318761121. doi: 10.1073/pnas.2318761121. Epub 2024 Jun 17. Proc Natl Acad Sci U S A. 2024. PMID: 38885389 Free PMC article.
-
Identification of a protein responsible for the synthesis of archaeal membrane-spanning GDGT lipids.Nat Commun. 2022 Mar 22;13(1):1545. doi: 10.1038/s41467-022-29264-x. Nat Commun. 2022. PMID: 35318330 Free PMC article.
-
Structural insights linking H-bridging of archaeal GDGTs to high temperature.Sci Total Environ. 2024 Oct 1;945:174120. doi: 10.1016/j.scitotenv.2024.174120. Epub 2024 Jun 18. Sci Total Environ. 2024. PMID: 38901598
-
One step beyond a ribosome: The ancient anaerobic core.Biochim Biophys Acta. 2016 Aug;1857(8):1027-1038. doi: 10.1016/j.bbabio.2016.04.284. Epub 2016 May 2. Biochim Biophys Acta. 2016. PMID: 27150504 Free PMC article. Review.
-
Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations.Microbiol Mol Biol Rev. 2007 Mar;71(1):97-120. doi: 10.1128/MMBR.00033-06. Microbiol Mol Biol Rev. 2007. PMID: 17347520 Free PMC article. Review.
Cited by
-
Discovering Hidden Archaeal and Bacterial Lipid Producers in a Euxinic Marine System.Environ Microbiol. 2025 Mar;27(3):e70054. doi: 10.1111/1462-2920.70054. Environ Microbiol. 2025. PMID: 40016913 Free PMC article.
-
A [2.1.0]-Fused Bicyclic Intermediate Is Produced during the Biosynthesis of Oxetane Nucleosides.J Am Chem Soc. 2025 Jul 23;147(29):25224-25232. doi: 10.1021/jacs.5c01831. Epub 2025 Jul 11. J Am Chem Soc. 2025. PMID: 40643284 Free PMC article.
-
Cyclization of archaeal membrane lipids impacts membrane protein activity and archaellum formation.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2423648122. doi: 10.1073/pnas.2423648122. Epub 2025 May 12. Proc Natl Acad Sci U S A. 2025. PMID: 40354536
-
Roles of mobile genetic elements and biosynthetic gene clusters in environmental adaptation of acidophilic archaeon Ferroplasma to extreme polluted environments.Front Microbiol. 2025 Jul 31;16:1654373. doi: 10.3389/fmicb.2025.1654373. eCollection 2025. Front Microbiol. 2025. PMID: 40822404 Free PMC article.
-
Initiation, Propagation, and Termination in the Chemistry of Radical SAM Enzymes.Biochemistry. 2024 Dec 17;63(24):3161-3183. doi: 10.1021/acs.biochem.4c00518. Epub 2024 Dec 3. Biochemistry. 2024. PMID: 39626071 Free PMC article. Review.
References
-
- Schouten S, Hopmans EC, Damsté JSS. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: a review. Org. Geochem. 2013;54:9–61. doi: 10.1016/j.orggeochem.2012.09.006. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

