The survival benefit of adjuvant trastuzumab with or without chemotherapy in the management of small (T1mic, T1a, T1b, T1c), node negative HER2+ breast cancer
- PMID: 38898072
- PMCID: PMC11187074
- DOI: 10.1038/s41523-024-00652-4
The survival benefit of adjuvant trastuzumab with or without chemotherapy in the management of small (T1mic, T1a, T1b, T1c), node negative HER2+ breast cancer
Abstract
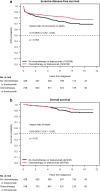
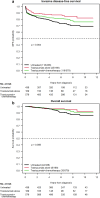
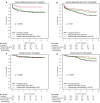
There is limited data regarding the added benefit of adjuvant systemic therapy in the management of small, node-negative, HER2+ breast cancer. In a multi-institutional retrospective analysis using the American Society of Clinical Oncology CancerLinQ database, we compared survival outcomes among T1a-c N0 HER2+ patients diagnosed between 2010 to 2021 who received locoregional therapy alone or in combination with adjuvant trastuzumab (+/- chemotherapy). Primary outcomes were invasive disease-free survival (iDFS) and overall survival (OS). Of the 1,184 patients, 436 received locoregional therapy alone. We found a statistically significant improvement in iDFS (HR 0.73, P = 0.003) and OS (HR 0.63, P = 0.023) on univariate analysis with adjuvant trastuzumab with or without chemotherapy which remained statistically significant on multivariate analysis. Three-arm univariate analysis found that iDFS was significantly improved with trastuzumab monotherapy (P = 0.003) and combination therapy (P = 0.027) compared to observation. Subgroup data suggests that T1b/c tumors derive the greatest benefit.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

