Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice
- PMID: 38898106
- PMCID: PMC11187144
- DOI: 10.1038/s41541-024-00899-9
Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice
Erratum in
-
Author Correction: Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice.NPJ Vaccines. 2024 Jul 9;9(1):125. doi: 10.1038/s41541-024-00917-w. NPJ Vaccines. 2024. PMID: 38982077 Free PMC article. No abstract available.
Abstract
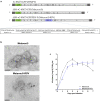
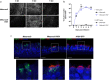
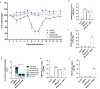
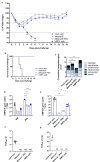
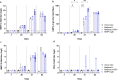
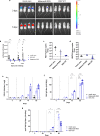
Live-Attenuated Vaccines (LAVs) stimulate robust mucosal and cellular responses and have the potential to protect against Respiratory Syncytial Virus (RSV) and Human Metapneumovirus (HMPV), the main etiologic agents of viral bronchiolitis and pneumonia in children. We inserted the RSV-F gene into an HMPV-based LAV (Metavac®) we previously validated for the protection of mice against HMPV challenge, and rescued a replicative recombinant virus (Metavac®-RSV), exposing both RSV- and HMPV-F proteins at the virion surface and expressing them in reconstructed human airway epithelium models. When administered to BALB/c mice by the intranasal route, bivalent Metavac®-RSV demonstrated its capacity to replicate with reduced lung inflammatory score and to protect against both RSV and lethal HMPV challenges in vaccinated mice while inducing strong IgG and broad RSV and HMPV neutralizing antibody responses. Altogether, our results showed the versatility of the Metavac® platform and suggested that Metavac®-RSV is a promising mucosal bivalent LAV candidate to prevent pneumovirus-induced diseases.
© 2024. The Author(s).
Conflict of interest statement
Manuel Rosa-Calatrava, Guy Boivin, Julia Dubois, and Marie-Eve Hamelin are co-founders and shareholders of Vaxxel SAS. Andrés Pizzorno and Olivier Terrier are shareholders of Vaxxel SAS. Julia Dubois was the R&D project manager of Vaxxel SAS. Caroline Chupin is an employee of Vaxxel SAS. The other authors declare no competing interests. The authors declare the following patent : EP22305240.2 – PCT/EP2023/055221 concerning Vaccine composition against two respiratory viruses (Inventors: Daniela Ogonczyk-Makowska, Jean-François Eléouët, Guy Boivin, Julia Dubois and Manuel Rosa-Calatrava ; Applicants : Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université Claude Bernard Lyon 1 (UCBL) Ecole Normale Supérieure de Lyon (ENS Lyon), Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE) and Vaxxel SAS).
Figures

References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986;140:543–546. - PubMed
Grants and funding
- 2020 0656/Association Nationale de la Recherche et de la Technologie (National Association for Research and Technology)
- 148361/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- AAP19 Metavac-T17/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources

