Principal component-based clinical aging clocks identify signatures of healthy aging and targets for clinical intervention
- PMID: 38898237
- PMCID: PMC11333290
- DOI: 10.1038/s43587-024-00646-8
Principal component-based clinical aging clocks identify signatures of healthy aging and targets for clinical intervention
Abstract
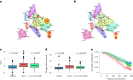
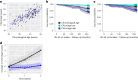
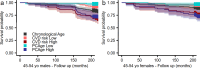
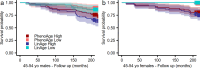
Clocks that measure biological age should predict all-cause mortality and give rise to actionable insights to promote healthy aging. Here we applied dimensionality reduction by principal component analysis to clinical data to generate a clinical aging clock (PCAge) identifying signatures (principal components) separating healthy and unhealthy aging trajectories. We found signatures of metabolic dysregulation, cardiac and renal dysfunction and inflammation that predict unsuccessful aging, and we demonstrate that these processes can be impacted using well-established drug interventions. Furthermore, we generated a streamlined aging clock (LinAge), based directly on PCAge, which maintains equivalent predictive power but relies on substantially fewer features. Finally, we demonstrate that our approach can be tailored to individual datasets, by re-training a custom clinical clock (CALinAge), for use in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) study of caloric restriction. Our analysis of CALERIE participants suggests that 2 years of mild caloric restriction significantly reduces biological age. Altogether, we demonstrate that this dimensionality reduction approach, through integrating different biological markers, can provide targets for preventative medicine and the promotion of healthy aging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

