The efficacy and safety of autologous epidermal cell suspensions for re-epithelialization of skin lesions: A systematic review and meta-analysis of randomized trials
- PMID: 38898373
- PMCID: PMC11186709
- DOI: 10.1111/srt.13820
The efficacy and safety of autologous epidermal cell suspensions for re-epithelialization of skin lesions: A systematic review and meta-analysis of randomized trials
Abstract
Background: Successful usage of autologous skin cell suspension (ASCS) has been demonstrated in some clinical trials. However, its efficacy and safety have not been verified. This latest systematic review and meta-analysis aim to examine the effects of autologous epidermal cell suspensions in re-epithelialization of skin lesions.
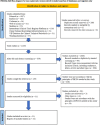
Methods: Relevant articles were retrieved from PubMed, Embase, Cochrane Database, Web of Science, International Clinical Trials Registry Platform, China National Knowledge Infrastructureris, VIP Database for Chinese Technical Periodicals and Wanfang database. The primary output measure was the healing time, and the secondary outputs were effective rate, size of donor site for treatment, size of study treatment area, operation time, pain scores, repigmentation, complications, scar scale scores and satisfaction scores. Data were pooled and expressed as relative risk (RR), mean difference (MD) and standardized mean difference (SMD) with a 95% confidence interval (CI).
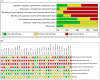
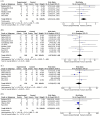
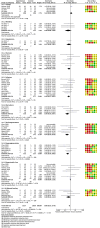
Results: Thirty-one studies were included in this systematic review and meta-analysis, with 914 patients who received autologous epidermal cell suspensions (treatment group) and 883 patients who received standard care or placebo (control group). The pooled data from all included studies demonstrated that the treatment group has significantly reduced healing time (SMD = -0.86; 95% CI: -1.59-0.14; p = 0.02, I2 = 95%), size of donar site for treatment (MD = -115.41; 95% CI: -128.74-102.09; p<0.001, I2 = 89%), operation time (MD = 25.35; 95% CI: 23.42-27.29; p<0.001, I2 = 100%), pain scores (SMD = -1.88; 95% CI: -2.86-0.90; p = 0.0002, I2 = 89%) and complications (RR = 0.59; 95% CI: 0.36-0.96; p = 0.03, I2 = 66%), as well as significantly increased effective rate (RR = 1.20; 95% CI: 1.01-1.42; p = 0.04, I2 = 77%). There were no significant differences in the size of study treatment area, repigmentation, scar scale scores and satisfaction scores between the two groups.
Conclusion: Our meta-analysis showed that autologous epidermal cell suspensions is beneficial for re-epithelialization of skin lesions as they significantly reduce the healing time, size of donar site for treatment, operation time, pain scores and complications, as well as increased effective rate. However, this intervention has minimal impact on size of treatment area, repigmentation, scar scale scores and satisfaction scores.
Keywords: autologous epidermal cell suspensions; complications; healing time; meta‐analysis; repigmentation; skin lesion; ulcer; vitiligo; wound.
© 2024 The Author(s). Skin Research and Technology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




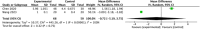
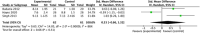
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

