Prolonged 14-day continuous infusion of high-dose ifosfamide for patients with relapsed and refractory high-grade osteosarcoma: a retrospective multicentre cohort study
- PMID: 38898388
- PMCID: PMC11186082
- DOI: 10.1186/s12885-024-12498-x
Prolonged 14-day continuous infusion of high-dose ifosfamide for patients with relapsed and refractory high-grade osteosarcoma: a retrospective multicentre cohort study
Abstract
Background: The prognosis of patients with Relapsed/Refractory Osteosarcoma (R/R OS) remains dismal without an agreement on systemic therapy. The use of High-Dose Ifosfamide (14 g/sqm) with an external pump in outpatient setting (14-IFO) in R/R OS patients is limited. This study represents the first retrospective cohort analysis focused on evaluating the activity and toxicity of 14-IFO in this setting.
Patients and methods: The study investigated 14-IFO activity, in terms of tumour response according to RECIST 1.1 criteria, as well as survival rates and toxicity, according to CTCAE v.5.
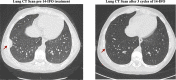
Results: The trial enrolled 26 patients with R/R OS. The Overall Response Rate (ORR) and Disease Control Rate (DCR) obtained was 23% and 57.5%, respectively. Patients with relapsed OS showed a higher ORR (45%) and DCR (82%) compared to refractory patients, irrespective of the number of prior treatment lines received. The achievement of disease control with 14-IFO administration enabled 27% of patients to undergo new local treatment. Four-month Progression-Free Survival (PFS) was 54% for all patients and 82% for the relapsed OS sub-group. Median Overall Survival (OSurv) was 13.7 months, with 1-year OSurv of 51% for all patients and 71% for relapsed patients. Age over 18 years and the presence of refractory disease were identified as negative prognostic factors for this patient cohort. A total of 101 cycles were evaluated for toxic assessment, demonstrating a tolerable profile without grade 3-4 non-haematological toxicities.
Conclusions: 14-IFO should be considered a viable treatment option for R/R OS, particularly due to its well tolerated toxicity profile and the potential for home-administration, which can improve patient quality of life without compromising efficacy.
Keywords: Chemotherapy; Ifosfamide; Osteosarcoma; Relapse.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors have declared no potential conflicts of interest.
Figures

References
-
- van Ewijk R, Herold N, Baecklund F, Baumhoer D, Boye K, Gaspar N, et al. European standard clinical practice recommendations for children and adolescents with primary and recurrent osteosarcoma. EJC Pediatr Oncol. 2023;2:100029. doi: 10.1016/j.ejcped.2023.100029. - DOI
-
- Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–408. doi: 10.1016/S1470-2045(16)30214-5. - DOI - PMC - PubMed
-
- Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, Doxorubicin, and cisplatin (MAP) plus maintenance Pegylated Interferon Alfa-2b versus MAP alone in patients with Resectable High-Grade Osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 Good Response Randomized Controlled Trial. JCO. 2015;33(20):2279–87. doi: 10.1200/JCO.2014.60.0734. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

