Efficient production of guanosine in Escherichia coli by combinatorial metabolic engineering
- PMID: 38898430
- PMCID: PMC11186194
- DOI: 10.1186/s12934-024-02452-8
Efficient production of guanosine in Escherichia coli by combinatorial metabolic engineering
Abstract
Background: Guanosine is a purine nucleoside that is widely used as a raw material for food additives and pharmaceutical products. Microbial fermentation is the main production method of guanosine. However, the guanosine-producing strains possess multiple metabolic pathway interactions and complex regulatory mechanisms. The lack of strains with efficiently producing-guanosine greatly limited industrial application.
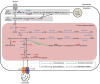
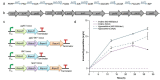
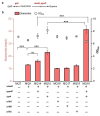
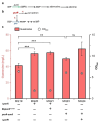
Results: We attempted to efficiently produce guanosine in Escherichia coli using systematic metabolic engineering. First, we overexpressed the purine synthesis pathway from Bacillus subtilis and the prs gene, and deleted three genes involved in guanosine catabolism to increase guanosine accumulation. Subsequently, we attenuated purA expression and eliminated feedback and transcription dual inhibition. Then, we modified the metabolic flux of the glycolysis and Entner-Doudoroff (ED) pathways and performed redox cofactors rebalancing. Finally, transporter engineering and enhancing the guanosine synthesis pathway further increased the guanosine titre to 134.9 mg/L. After 72 h of the fed-batch fermentation in shake-flask, the guanosine titre achieved 289.8 mg/L.
Conclusions: Our results reveal that the guanosine synthesis pathway was successfully optimized by combinatorial metabolic engineering, which could be applicable to the efficient synthesis of other nucleoside products.
Keywords: Escherichia coli; Guanosine; Integration expression; Metabolic engineering; Metabolic flux.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The metabolic engineering of Escherichia coli for the high-yield production of hypoxanthine.Microb Cell Fact. 2024 Nov 14;23(1):309. doi: 10.1186/s12934-024-02576-x. Microb Cell Fact. 2024. PMID: 39543621 Free PMC article.
-
Characterization of genome-reduced Bacillus subtilis strains and their application for the production of guanosine and thymidine.Microb Cell Fact. 2016 Jun 3;15:94. doi: 10.1186/s12934-016-0494-7. Microb Cell Fact. 2016. PMID: 27260256 Free PMC article.
-
Metabolic engineering of Escherichia coli for high-yield uridine production.Metab Eng. 2018 Sep;49:248-256. doi: 10.1016/j.ymben.2018.09.001. Epub 2018 Sep 4. Metab Eng. 2018. PMID: 30189293
-
Microbial Synthesis of Nucleosides: Advances and Prospects.ACS Synth Biol. 2025 Jan 17;14(1):1-9. doi: 10.1021/acssynbio.4c00530. Epub 2024 Dec 12. ACS Synth Biol. 2025. PMID: 39665672 Review.
-
Advances and prospects in metabolic engineering of Escherichia coli for L-tryptophan production.World J Microbiol Biotechnol. 2022 Jan 6;38(2):22. doi: 10.1007/s11274-021-03212-1. World J Microbiol Biotechnol. 2022. PMID: 34989926 Review.
Cited by
-
5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis.Microorganisms. 2025 Mar 31;13(4):792. doi: 10.3390/microorganisms13040792. Microorganisms. 2025. PMID: 40284629 Free PMC article.
-
Increased distribution of carbon metabolic flux during de novo cytidine biosynthesis via attenuation of the acetic acid metabolism pathway in Escherichia coli.Microb Cell Fact. 2025 Feb 4;24(1):36. doi: 10.1186/s12934-025-02657-5. Microb Cell Fact. 2025. PMID: 39905471 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

