Tumor microenvironment responsive nano-herb and CRISPR delivery system for synergistic chemotherapy and immunotherapy
- PMID: 38898493
- PMCID: PMC11186293
- DOI: 10.1186/s12951-024-02571-9
Tumor microenvironment responsive nano-herb and CRISPR delivery system for synergistic chemotherapy and immunotherapy
Abstract
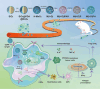
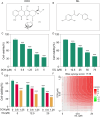
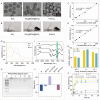
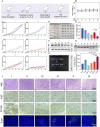
Chemoresistance remains a significant challenge for effective breast cancer treatment which leads to cancer recurrence. CRISPR-directed gene editing becomes a powerful tool to reduce chemoresistance by reprogramming the tumor microenvironment. Previous research has revealed that Chinese herbal extracts have significant potential to overcome tumor chemoresistance. However, the therapeutic efficacy is often limited due to their poor tumor targeting and in vivo durability. Here we have developed a tumor microenvironment responsive nanoplatform (H-MnO2(ISL + DOX)-PTPN2@HA, M(I + D)PH) for nano-herb and CRISPR codelivery to reduce chemoresistance. Synergistic tumor inhibitory effects were achieved by the treatment of isoliquiritigenin (ISL) with doxorubicin (DOX), which were enhanced by CRISPR-based gene editing to target protein tyrosine phosphatase non-receptor type 2 (PTPN2) to initiate long-term immunotherapy. Efficient PTPN2 depletion was observed after treatment with M(I + D)PH nanoparticles, which resulted in the recruitment of intratumoral infiltrating lymphocytes and an increase of proinflammatory cytokines in the tumor tissue. Overall, our nanoparticle platform provides a diverse technique for accomplishing synergistic chemotherapy and immunotherapy, which offers an effective treatment alternative for malignant neoplasms.
Keywords: CRISPR-Cas 9; Doxorubicin (DOX); Immunotherapy; Isoliquiritigenin (ISL); Protein tyrosine phosphatase non-receptor type 2 (PTPN2); Synergistic therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Bioorthogonal Reaction-Mediated Tumor-Selective Delivery of CRISPR/Cas9 System for Dual-Targeted Cancer Immunotherapy.Angew Chem Int Ed Engl. 2023 Sep 11;62(37):e202306863. doi: 10.1002/anie.202306863. Epub 2023 Aug 3. Angew Chem Int Ed Engl. 2023. PMID: 37485554
-
Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy.Acta Biomater. 2018 Jan 15;66:310-324. doi: 10.1016/j.actbio.2017.11.010. Epub 2017 Nov 10. Acta Biomater. 2018. PMID: 29129789
-
Combining Tumor Microenvironment Modulating Nanoparticles with Doxorubicin to Enhance Chemotherapeutic Efficacy and Boost Antitumor Immunity.J Natl Cancer Inst. 2019 Apr 1;111(4):399-408. doi: 10.1093/jnci/djy131. J Natl Cancer Inst. 2019. PMID: 30239773
-
Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy.Adv Drug Deliv Rev. 2021 Jan;168:158-180. doi: 10.1016/j.addr.2020.04.010. Epub 2020 May 1. Adv Drug Deliv Rev. 2021. PMID: 32360576 Review.
-
Genetic reprogramming for NK cell cancer immunotherapy with CRISPR/Cas9.Immunology. 2019 Oct;158(2):63-69. doi: 10.1111/imm.13094. Epub 2019 Aug 14. Immunology. 2019. PMID: 31315144 Free PMC article. Review.
Cited by
-
Oxygen-Generating Biomimetic Nano-Herb System for Synergistic Therapy & Pain Relief in Triple-Negative Breast Cancer via HIF-1α/VEGF Pathway.Int J Nanomedicine. 2025 May 31;20:7113-7132. doi: 10.2147/IJN.S523754. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40470112 Free PMC article.
-
Protein tyrosine phosphatase nonreceptor 2: A New biomarker for digestive tract cancers.World J Gastrointest Oncol. 2025 Feb 15;17(2):100546. doi: 10.4251/wjgo.v17.i2.100546. World J Gastrointest Oncol. 2025. PMID: 39958541 Free PMC article.
-
Recent advances in self-targeting natural product-based nanomedicines.J Nanobiotechnology. 2025 Jan 20;23(1):31. doi: 10.1186/s12951-025-03092-9. J Nanobiotechnology. 2025. PMID: 39833846 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- ZKX23037/Key Project of Nanjing Medical Science and Technology Development Project
- RCZD202302/Key Project of Talent Support Plan of Nanjing Second Hospital
- M2020055/the medical scientific research project of Jiangsu Health Commission
- XRZ2021078/Nanjing University of Traditional Chinese Medicine Natural Science Foundation
- 82203337/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials

