FBS-based cryoprotective compositions for effective cryopreservation of gut microbiota and key intestinal microorganisms
- PMID: 38898515
- PMCID: PMC11188276
- DOI: 10.1186/s13104-024-06836-2
FBS-based cryoprotective compositions for effective cryopreservation of gut microbiota and key intestinal microorganisms
Abstract
Objective: The need for innovative techniques to preserve microbiota for extended periods, while maintaining the species composition and quantitative balance of the bacterial community, is becoming increasingly important. To address this need, we propose an efficient approach to cryopreserve human gut microbiota using a two-component cryoprotective composition comprising fetal bovine serum (FBS) and 5% dimethyl sulfoxide (DMSO). Fetal serum is a commonly utilized component in the freezing media for eukaryotic cells, however, its effects on prokaryotic cells have not been extensively researched.
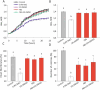
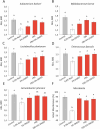
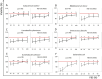
Results: In our study, we demonstrated the high efficiency of using a two-component cryoprotective medium, FBS + 5% DMSO, for cryopreservation of human gut microbiota using three different methods. According to the obtained results, the intact donor microbiota was preserved at a level of 85 ± 4% of the initial composition based on fluorescent analysis using the LIVE/DEAD test. No differences in survival were observed when comparing with pure DMSO and FBS media. The photometric measurement method for growth of aerobic bacteria (A. johnsoni), facultative anaerobes (E. coli, E. faecalis), microaerophilic (L. plantarum), and obligate anaerobic bacterial cultures (E. barkeri, B. breve) also demonstrated high viability rates of 94-98% in the two-component protective medium, reaching intact control levels. However, for anaerobic microflora representatives, serum proved to be a more suitable cryoprotectant. Also, we demonstrated that using cryoprotective media with 50-75% FBS content is enough to preserve a significant level of bacterial cell viability, from an economic standpoint.
Keywords: Bacterial cultures; Cryopreservation; Fetal bovine serum; Gut microbiota.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that have no competing interests.
Figures
Similar articles
-
Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells.Biopreserv Biobank. 2014 Apr;12(2):99-105. doi: 10.1089/bio.2013.0078. Biopreserv Biobank. 2014. PMID: 24749876 Free PMC article.
-
Evaluation of human platelet lysate and dimethyl sulfoxide as cryoprotectants for the cryopreservation of human adipose-derived stem cells.Biochem Biophys Res Commun. 2017 Sep 9;491(1):198-203. doi: 10.1016/j.bbrc.2017.07.076. Epub 2017 Jul 13. Biochem Biophys Res Commun. 2017. PMID: 28712869
-
Combining dimethyl sulphoxide (DMSO) with different cryoprotectants ensures better cartilage cell cryopreservation.Cryo Letters. 2021 Jul-Aug;42(4):220-226. Cryo Letters. 2021. PMID: 35363841
-
Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity?Regen Med. 2020 Mar;15(3):1463-1491. doi: 10.2217/rme-2019-0145. Epub 2020 Apr 28. Regen Med. 2020. PMID: 32342730 Review.
-
Strategies to improve platelet cryopreservation: A narrative review.Transfusion. 2025 Apr;65(4):740-749. doi: 10.1111/trf.18204. Epub 2025 Mar 10. Transfusion. 2025. PMID: 40059666 Free PMC article. Review. No abstract available.
References
-
- Matsuki T, Tanaka R. Function of the human gut microbiota. THuman Microbiota Microbiome. 2014;90:26–33. doi: 10.1079/9781780640495.0090. - DOI
-
- Ermolenko EI, Suvorov AN, Simanenkov VI, Sundukova ZR, Tsapieva AN, Solovyov OI. Method for producing autoprobiotic of Enterocuccus faecium being representative of indigenic host intestinal microflora. Patent 2460778 C1. 2010. RU.
-
- Alexander MT, Simione FP. Factors affecting the recovery of freeze-dried Saccharomyces cerevisiae. Abstr. of the Annual Meeting of the American Society for Microbiology. 1980; pp. 86.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

