Advancing fluorescence imaging: enhanced control of cyanine dye-doped silica nanoparticles
- PMID: 38898529
- PMCID: PMC11188253
- DOI: 10.1186/s12951-024-02638-7
Advancing fluorescence imaging: enhanced control of cyanine dye-doped silica nanoparticles
Abstract
Background: Silica nanoparticles (SNPs) have immense potential in biomedical research, particularly in drug delivery and imaging applications, owing to their stability and minimal interactions with biological entities such as tissues or cells.
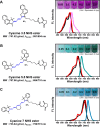
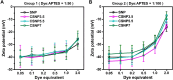
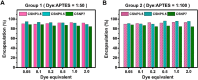
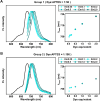
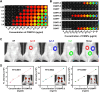
Results: With synthesized and characterized cyanine-dye-doped fluorescent SNPs (CSNPs) using cyanine 3.5, 5.5, and 7 (Cy3.5, Cy5.5, and Cy7). Through systematic analysis, we discerned variations in the surface charge and fluorescence properties of the nanoparticles contingent on the encapsulated dye-(3-aminopropyl)triethoxysilane conjugate, while their size and shape remained constant. The fluorescence emission spectra exhibited a redshift correlated with increasing dye concentration, which was attributed to cascade energy transfer and self-quenching effects. Additionally, the fluorescence signal intensity showed a linear relationship with the particle concentration, particularly at lower dye equivalents, indicating a robust performance suitable for imaging applications. In vitro assessments revealed negligible cytotoxicity and efficient cellular uptake of the nanoparticles, enabling long-term tracking and imaging. Validation through in vivo imaging in mice underscored the versatility and efficacy of CSNPs, showing single-switching imaging capabilities and linear signal enhancement within subcutaneous tissue environment.
Conclusions: This study provides valuable insights for designing fluorescence imaging and optimizing nanoparticle-based applications in biomedical research, with potential implications for targeted drug delivery and in vivo imaging of tissue structures and organs.
Keywords: Characterization; Cyanine N-hydroxysuccinimide ester; Fluorescence in vitro and in vivo image; Imaging optimization; Silica nanoparticle.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Ma D, Kell AJ, Tan S, Jakubek ZJ, Simard B. Photophysical properties of dye-doped silica nanoparticles bearing different types of dye-silica interactions. J Phys Chem C. 2009;113:15974–15981. doi: 10.1021/jp905812f. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

