Sex-specific differences in the mechanisms for enhanced thromboxane A2-mediated vasoconstriction in adult offspring exposed to prenatal hypoxia
- PMID: 38898532
- PMCID: PMC11188502
- DOI: 10.1186/s13293-024-00627-x
Sex-specific differences in the mechanisms for enhanced thromboxane A2-mediated vasoconstriction in adult offspring exposed to prenatal hypoxia
Abstract
Background: Prenatal hypoxia, a common pregnancy complication, leads to impaired cardiovascular outcomes in the adult offspring. It results in impaired vasodilation in coronary and mesenteric arteries of the adult offspring, due to reduced nitric oxide (NO). Thromboxane A2 (TxA2) is a potent vasoconstrictor increased in cardiovascular diseases, but its role in the impact of prenatal hypoxia is unknown. To prevent the risk of cardiovascular disease by prenatal hypoxia, we have tested a maternal treatment using a nanoparticle-encapsulated mitochondrial antioxidant (nMitoQ). We hypothesized that prenatal hypoxia enhances vascular TxA2 responses in the adult offspring, due to decreased NO modulation, and that this might be prevented by maternal nMitoQ treatment.
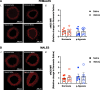
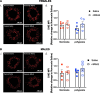
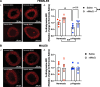
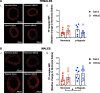
Methods: Pregnant Sprague-Dawley rats received a single intravenous injection (100 µL) of vehicle (saline) or nMitoQ (125 µmol/L) on gestational day (GD)15 and were exposed to normoxia (21% O2) or hypoxia (11% O2) from GD15 to GD21 (term = 22 days). Coronary and mesenteric arteries were isolated from the 4-month-old female and male offspring, and vasoconstriction responses to U46619 (TxA2 analog) were evaluated using wire myography. In mesenteric arteries, L-NAME (pan-NO synthase (NOS) inhibitor) was used to assess NO modulation. Mesenteric artery endothelial (e)NOS, and TxA2 receptor expression, superoxide, and 3-nitrotyrosine levels were assessed by immunofluorescence.
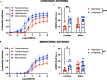
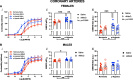
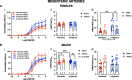
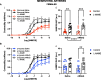
Results: Prenatal hypoxia resulted in increased U46619 responsiveness in coronary and mesenteric arteries of the female offspring, and to a lesser extent in the male offspring, which was prevented by nMitoQ. In females, there was a reduced impact of L-NAME in mesenteric arteries of the prenatal hypoxia saline-treated females, and reduced 3-nitrotyrosine levels. In males, L-NAME increased U46619 responses in mesenteric artery to a similar extent, but TxA2 receptor expression was increased by prenatal hypoxia. There were no changes in eNOS or superoxide levels.
Conclusions: Prenatal hypoxia increased TxA2 vasoconstrictor capacity in the adult offspring in a sex-specific manner, via reduced NO modulation in females and increased TP expression in males. Maternal placental antioxidant treatment prevented the impact of prenatal hypoxia. These findings increase our understanding of how complicated pregnancies can lead to a sex difference in the programming of cardiovascular disease in the adult offspring.
Keywords: Coronary arteries; Developmental origins of health and disease; Mesenteric arteries; Nitric oxide; Placental treatment; Pregnancy complications; Prenatal hypoxia; Sex differences; Thromboxane A2; nMitoQ.
Plain language summary
Prenatal hypoxia, when the fetus does not receive enough oxygen, is a common problem during pregnancy that impacts the developing fetus. It is associated with an increased risk of cardiovascular disease in the offspring in adulthood. While the mechanisms are not fully understood, the blood vessel function in the offspring may be impacted by prenatal hypoxia. We hypothesize that prenatal hypoxia increases the constriction of the blood vessels in the offspring. The placenta, an essential organ for fetal development, supplies oxygen and nutrients to the fetus. In prenatal hypoxia pregnancies, the placenta does not work properly. We have been studying a placental treatment (called nMitoQ) to improve placenta function and thereby the blood vessel function of the offspring. We used a rat model of prenatal hypoxia, where pregnant rats (dams) were placed in a low oxygen environment (hypoxia) during the last trimester of pregnancy. Control rats were kept in normal oxygen conditions. The dams were treated with nMitoQ, or with saline (control). Next, we studied the blood vessels of the offspring in adulthood. We found that prenatal hypoxia increases the constriction of the blood vessels, which was prevented by treating the dams with nMitoQ. Interestingly, this impact was more severe in females compared to males, and the mechanisms were different between the sexes. This study helps in the understanding of how complicated pregnancies can impair cardiovascular health in the offspring, and in a potential development of targeted and sex-specific therapies for those offspring at high risk for future cardiovascular disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

