Knockdown of ATRX enhances radiosensitivity in glioblastoma
- PMID: 38898533
- PMCID: PMC11186225
- DOI: 10.1186/s41016-024-00371-6
Knockdown of ATRX enhances radiosensitivity in glioblastoma
Abstract
Background: Glioblastoma are highly malignant type of primary brain tumors. Treatment for glioblastoma multiforme (GBM) generally involves surgery combined with chemotherapy and radiotherapy. However, the development of tumoral chemo- and radioresistance induces complexities in clinical practice. Multiple signaling pathways are known to be involved in radiation-induced cell survival. However, the role of alpha-thalassemia X-linked mutant retardation syndrome (ATRX), a chromatin remodeling protein, in GBM radioresistance remains unclear.
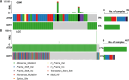
Methods: In the present study, the ATRX mutation rate in patients with glioma was obtained from The Cancer Genome Atlas, while its expression analyzed using bioinformatics. Datasets were also obtained from the Gene Expression Omnibus, and ATRX expression levels following irradiation of GBM were determined. The effects of ATRX on radiosensitivity were investigated using a knockdown assays.
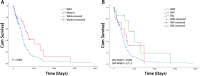
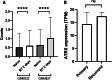
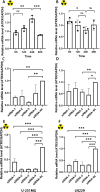
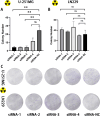
Results: The present study demonstrated that the ATRX mutation rate in patients with GBM was significantly lower than that in patients with low-grade glioma, and that patients harboring an ATRX mutation exhibited a prolonged survival, compared with to those harboring the wild-type gene. Single-cell RNA sequencing demonstrated that ATRX counts increased 2 days after irradiation, with ATRX expression levels also increasing in U-251MG radioresistant cells. Moreover, the results of in vitro irradiation assays revealed that ATRX expression was increased in U-251MG cells, while ATRX knockdown was associated with increased levels of radiosensitivity.
Conclusions: High ATRX expression levels in primary GBM may contribute to high levels of radioresistance. Thus ATRX is a potential target for overcoming the radioresistance in GBM.
Keywords: ATRX; Glioblastoma; Knockdown; Radiosensitivity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
