Internal and external validation of indocyanine green plasma disappearance rate to discard liver grafts before procurement
- PMID: 38898570
- PMCID: PMC11599826
- DOI: 10.14701/ahbps.24-086
Internal and external validation of indocyanine green plasma disappearance rate to discard liver grafts before procurement
Abstract
Backgrounds/aims: Thirty percent of liver grafts in donors after brain death (DBD) in Spain are rejected by procurement surgeons owing to marginal graft quality. Poor donor indocyanine green (ICG) clearance has been associated with graft discard and malfunction. This study aimed to internally and externally validate the predictive value of ICG-plasma disappearance rate (ICG-PDR) to reject grafts before donation and set a cut-off to avoid missing any potential effective donors.
Methods: Between March 2017 and August 2023, ICG clearance test was performed immediately before procurement in 71 DBD. The surgeon was blinded to test results. Univariate and multivariate analyses were performed to detect independent predictors of graft discard. Discrimination and calibration of predictors were assessed and a cut-off with 100% specificity was set. External validation was performed on 17 donors evaluated by three other transplantation teams.
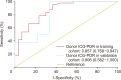
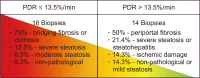
Results: In the training cohort, 30 of 71 grafts were discarded for transplantation. ICG-PDR was the only donor variable independently associated with graft discard. The area under receiver operating characteristic curve for ICG-PDR was 0.875 (95% confidence interval: 0.768-0.947) and good calibration was observed. Below a PDR of 13.5%/min, no graft was accepted for transplantation. These results were successfully validated using the external cohort of donors.
Conclusions: ICG clearance test performed in DBD was internally and externally validated to predict liver graft discard. It could be used as a screening tool before donation to avoid unnecessary costs of travel and human resources.
Keywords: Brain death; Delayed graft function; Donor selection; Indocyanine green; Spain.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Organización Nacional de Trasplantes (ONT), author Actividad de donación y trasplante hepático España 2022 [Internet] ONT; 2022. [cited 2024 Jan 23]. Available from: https://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-HEPA... .
LinkOut - more resources
Full Text Sources
