The multifaceted functions of DNA-PKcs: implications for the therapy of human diseases
- PMID: 38898995
- PMCID: PMC11185949
- DOI: 10.1002/mco2.613
The multifaceted functions of DNA-PKcs: implications for the therapy of human diseases
Abstract
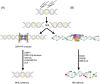
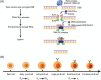
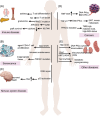
The DNA-dependent protein kinase (DNA-PK), catalytic subunit, also known as DNA-PKcs, is complexed with the heterodimer Ku70/Ku80 to form DNA-PK holoenzyme, which is well recognized as initiator in the nonhomologous end joining (NHEJ) repair after double strand break (DSB). During NHEJ, DNA-PKcs is essential for both DNA end processing and end joining. Besides its classical function in DSB repair, DNA-PKcs also shows multifaceted functions in various biological activities such as class switch recombination (CSR) and variable (V) diversity (D) joining (J) recombination in B/T lymphocytes development, innate immunity through cGAS-STING pathway, transcription, alternative splicing, and so on, which are dependent on its function in NHEJ or not. Moreover, DNA-PKcs deficiency has been proven to be related with human diseases such as neurological pathogenesis, cancer, immunological disorder, and so on through different mechanisms. Therefore, it is imperative to summarize the latest findings about DNA-PKcs and diseases for better targeting DNA-PKcs, which have shown efficacy in cancer treatment in preclinical models. Here, we discuss the multifaceted roles of DNA-PKcs in human diseases, meanwhile, we discuss the progresses of DNA-PKcs inhibitors and their potential in clinical trials. The most updated review about DNA-PKcs will hopefully provide insights and ideas to understand DNA-PKcs associated diseases.
Keywords: DNA damage; DNA‐PKcs; V(D)J recombination; class switch recombination; innate immunity.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Functional redundancy between the XLF and DNA-PKcs DNA repair factors in V(D)J recombination and nonhomologous DNA end joining.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2234-9. doi: 10.1073/pnas.1222573110. Epub 2013 Jan 23. Proc Natl Acad Sci U S A. 2013. PMID: 23345432 Free PMC article.
-
Artemis-independent functions of DNA-dependent protein kinase in Ig heavy chain class switch recombination and development.Proc Natl Acad Sci U S A. 2005 Feb 15;102(7):2471-5. doi: 10.1073/pnas.0409857102. Epub 2005 Feb 7. Proc Natl Acad Sci U S A. 2005. PMID: 15699324 Free PMC article.
-
Phosphorylation of DNA-PKcs at the S2056 cluster ensures efficient and productive lymphocyte development in XLF-deficient mice.Proc Natl Acad Sci U S A. 2023 Jun 20;120(25):e2221894120. doi: 10.1073/pnas.2221894120. Epub 2023 Jun 12. Proc Natl Acad Sci U S A. 2023. PMID: 37307443 Free PMC article.
-
DNA-Dependent Protein Kinase Catalytic Subunit: The Sensor for DNA Double-Strand Breaks Structurally and Functionally Related to Ataxia Telangiectasia Mutated.Genes (Basel). 2021 Jul 27;12(8):1143. doi: 10.3390/genes12081143. Genes (Basel). 2021. PMID: 34440313 Free PMC article. Review.
-
DNA-Dependent Protein Kinase Catalytic Subunit (DNA-PKcs): Beyond the DNA Double-Strand Break Repair.Mol Cells. 2023 Apr 30;46(4):200-205. doi: 10.14348/molcells.2023.2164. Epub 2023 Feb 9. Mol Cells. 2023. PMID: 36756777 Free PMC article. Review.
Cited by
-
Optimizing predictive accuracy of the two-component repair model for pediatric TBI: Pediatric-specific parameters, toxicity endpoint harmonization, and dose-rate safeguards.Clin Transl Radiat Oncol. 2025 Jun 27;54:101004. doi: 10.1016/j.ctro.2025.101004. eCollection 2025 Sep. Clin Transl Radiat Oncol. 2025. PMID: 40673134 Free PMC article.
-
Medicinal chemistry breakthroughs on ATM, ATR, and DNA-PK inhibitors as prospective cancer therapeutics.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2489720. doi: 10.1080/14756366.2025.2489720. Epub 2025 Apr 21. J Enzyme Inhib Med Chem. 2025. PMID: 40256842 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
