Robust battery interphases from dilute fluorinated cations
- PMID: 38899028
- PMCID: PMC11185048
- DOI: 10.1039/d4ee00296b
Robust battery interphases from dilute fluorinated cations
Abstract
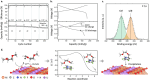
Controlling solid electrolyte interphase (SEI) in batteries is crucial for their efficient cycling. Herein, we demonstrate an approach to enable robust battery performance that does not rely on high fractions of fluorinated species in electrolytes, thus substantially decreasing the environmental footprint and cost of high-energy batteries. In this approach, we use very low fractions of readily reducible fluorinated cations in electrolyte (∼0.1 wt%) and employ electrostatic attraction to generate a substantial population of these cations at the anode surface. As a result, we can form a robust fluorine-rich SEI that allows for dendrite-free deposition of dense Li and stable cycling of Li-metal full cells with high-voltage cathodes. Our approach represents a general strategy for delivering desired chemical species to battery anodes through electrostatic attraction while using minute amounts of additive.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have a patent (US patent provisional application number 63398320) related to the electrolytes described in this article.
Figures



References
-
- Liu J. Bao Z. Cui Y. Dufek E. J. Goodenough J. B. Khalifah P. Li Q. Liaw B. Y. Liu P. Manthiram A. Meng Y. S. Subramanian V. R. Toney M. F. Viswanathan V. V. Whittingham M. S. Xiao J. Xu W. Yang J. Yang X.-Q. Zhang J.-G. Nat. Energy. 2019;4:180–186. doi: 10.1038/s41560-019-0338-x. - DOI
-
- Louli A. J. Eldesoky A. Weber R. Genovese M. Coon M. deGooyer J. Deng Z. White R. T. Lee J. Rodgers T. Petibon R. Hy S. Cheng S. J. H. Dahn J. R. Nat. Energy. 2020;5:693–702. doi: 10.1038/s41560-020-0668-8. - DOI
-
- Chen K.-H. Wood K. N. Kazyak E. LePage W. S. Davis A. L. Sanchez A. J. Dasgupta N. P. J. Mater. Chem. A. 2017;5:11671–11681. doi: 10.1039/C7TA00371D. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
