Insights into the sustainability of liquid chromatographic methods for favipiravir bioanalysis: a comparative study
- PMID: 38899032
- PMCID: PMC11185049
- DOI: 10.1039/d4ra03017f
Insights into the sustainability of liquid chromatographic methods for favipiravir bioanalysis: a comparative study
Abstract
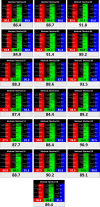
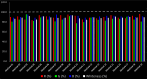
The introduction of favipiravir as a broad-spectrum antiviral agent, particularly in treating influenza and exploring its potential against COVID-19, emphasizes the necessity for efficient analytical methods. Liquid chromatography has emerged as a commonly utilized technique for quantifying favipiravir in biological fluids. However, the environmental and health concerns linked to classical analytical methods mean a transition toward green analytical chemistry is required. This study investigates the environmental impact of 19 liquid chromatographic methods utilized in the bioanalysis of favipiravir. Recognizing the importance of eco-friendly practices in pharmaceutical analysis, the study employs three widely accepted greenness assessment tools: Analytical Eco-Scale (AES), Green Analytical Procedure Index (GAPI), and Analytical Greenness Calculator (AGREE). Moreover, it incorporates a comprehensive evaluation on a global scale utilizing the whiteness assessment tool Red-Green-Blue 12 (RGB 12). The comprehensive evaluation aims to extend beyond traditional validation criteria and considerations of green chemistry, providing insights into the development of practically efficient, eco-friendly and economical analytical methods for favipiravir determination. This study emphasizes the necessity of planning for the environmental impact and overall sustainability of analytical methods before laboratory trials. Additionally, the integration of greenness/whiteness evaluation in method validation protocols is strongly advocated, emphasizing the importance of critical and global evaluations in analytical chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
The ecological impact of liquid chromatographic methods reported for bioanalysis of COVID-19 drug, hydroxychloroquine: Insights on greenness assessment.Microchem J. 2023 Jan;184:108145. doi: 10.1016/j.microc.2022.108145. Epub 2022 Nov 9. Microchem J. 2023. PMID: 36404889 Free PMC article.
-
Analytical Quality by Design-Assisted Eco-Friendly RP-HPLC Method for the Simultaneous Estimation of Artificial Sweeteners in Commercial Food Samples Utilizing a Green Ultrasound-Assisted Extraction Technique: Greenness, Blueness, and Whiteness Appraisal.J AOAC Int. 2025 Jan 1;108(1):10-22. doi: 10.1093/jaoacint/qsae085. J AOAC Int. 2025. PMID: 39514010
-
Environmental impact of the reported chromatographic methods for the determination of the first FDA-Approved therapy for COVID-19 Patients, Remdesivir: A comparative study.Microchem J. 2022 May;176:107242. doi: 10.1016/j.microc.2022.107242. Epub 2022 Jan 30. Microchem J. 2022. PMID: 35125520 Free PMC article.
-
Green analytical chemistry metrics for evaluating the greenness of analytical procedures.J Pharm Anal. 2024 Nov;14(11):101013. doi: 10.1016/j.jpha.2024.101013. Epub 2024 May 25. J Pharm Anal. 2024. PMID: 39759968 Free PMC article. Review.
-
Current green capillary electrophoresis and liquid chromatography methods for analysis of pharmaceutical and biomedical samples (2019-2023) - A review.Anal Chim Acta. 2024 Sep 22;1323:342889. doi: 10.1016/j.aca.2024.342889. Epub 2024 Jun 20. Anal Chim Acta. 2024. PMID: 39182966 Review.
Cited by
-
A novel LC-TQ-MS/MS method for quantifying mefenamic acid-NDSRI (N-nitroso drug substance-related impurity) in mefenamic acid tablet and pediatric suspension dosage forms: a comparative study with a cost-effective white, green, and blue UPLC method.RSC Adv. 2025 Jan 21;15(3):1957-1969. doi: 10.1039/d4ra08425j. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39839227 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

