Direction-oriented fiber guiding with a tunable tri-layer-3D scaffold for periodontal regeneration
- PMID: 38899033
- PMCID: PMC11186324
- DOI: 10.1039/d4ra01459f
Direction-oriented fiber guiding with a tunable tri-layer-3D scaffold for periodontal regeneration
Abstract
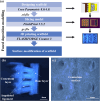
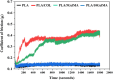
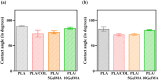
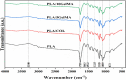
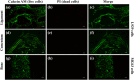
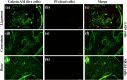
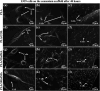
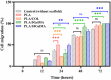
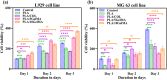
Multilayered scaffolds mimicking mechanical and biological host tissue architectures are the current prerequisites for successful tissue regeneration. We propose our tunable tri-layered scaffold, designed to represent the native periodontium for potential regenerative applications. The fused deposition modeling platform is used to fabricate the novel movable three-layered polylactic acid scaffold mimicking in vivo cementum, periodontal ligament, and alveolar bone layers. The scaffold is further provided with multiple angulated fibers, offering directional guidance and facilitating the anchorage dependence on cell adhesion. Additionally, surface modifications of the scaffold were made by incorporating coatings like collagen and different concentrations of gelatin methacryloyl to enrich the cell adhesion and proliferation. The surface characterization of our designed scaffolds was performed using tribological studies, atomic force microscopy, contact angle measurement, scanning electron microscopy, and micro-computed tomography. Furthermore, the material characterization of this scaffold was investigated by attenuated total reflectance-Fourier transformed infrared spectroscopy. The scaffold's mechanical characterization, such as strength and compression modulus, was demonstrated by compression testing. The L929 mouse fibroblast cells and MG63 human osteosarcoma cells have been cultured on the scaffold. The scaffold's superior biocompatibility was evaluated using fluorescence dye with fluorescence microscopy, scanning electron microscopy, in vitro wound healing assay, MTT assay, and flow cytometry. The mineralization capability of the scaffolds was also studied. In conclusion, our study demonstrated the construction of a multilayered movable scaffold, which is highly biocompatible and most suitable for various downstream applications such as periodontium and in situ tissue regeneration of complex, multilayered tissues.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium.Acta Biomater. 2015 Oct;25:240-52. doi: 10.1016/j.actbio.2015.07.023. Epub 2015 Jul 15. Acta Biomater. 2015. PMID: 26188325
-
Functionally gradient three-dimensional graphene foam-based polymeric scaffolds for multilayered tissue regeneration.RSC Adv. 2023 Jan 4;13(2):1245-1255. doi: 10.1039/d2ra06018c. eCollection 2023 Jan 3. RSC Adv. 2023. PMID: 36686898 Free PMC article.
-
Engineering poly(hydroxy butyrate-co-hydroxy valerate) based vascular scaffolds to mimic native artery.Int J Biol Macromol. 2018 Apr 1;109:85-98. doi: 10.1016/j.ijbiomac.2017.12.077. Epub 2017 Dec 13. Int J Biol Macromol. 2018. PMID: 29247731
-
Bone Scaffold Materials in Periodontal and Tooth-supporting Tissue Regeneration: A Review.Curr Stem Cell Res Ther. 2024;19(4):449-460. doi: 10.2174/1574888X18666221227142055. Curr Stem Cell Res Ther. 2024. PMID: 36578254 Review.
-
Layered scaffolds in periodontal regeneration.J Oral Biol Craniofac Res. 2022 Nov-Dec;12(6):782-797. doi: 10.1016/j.jobcr.2022.09.001. Epub 2022 Sep 13. J Oral Biol Craniofac Res. 2022. PMID: 36159068 Free PMC article. Review.
Cited by
-
The comprehensive progress of tooth regeneration from the tooth development to tissue engineering and clinical application.Cell Regen. 2025 Jul 31;14(1):33. doi: 10.1186/s13619-025-00249-7. Cell Regen. 2025. PMID: 40742495 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

