Insight into the liposomal encapsulation of mono and bis-naphthalimides
- PMID: 38899150
- PMCID: PMC11185046
- DOI: 10.1039/d3pm00060e
Insight into the liposomal encapsulation of mono and bis-naphthalimides
Abstract
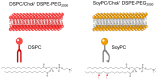
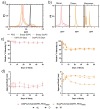
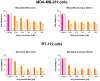
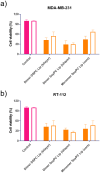
Mitonafide-loaded liposomes are a promising strategy to overcome the neurotoxicity observed in clinical trials for this drug. This study investigates the influence of loaded mitonafide or a dimer analogue on different liposomal formulations and their therapeutic efficacy in vitro. Physicochemical properties of the liposomes were manipulated using different loading methods (namely bilayer or core loading) and varying the rigidity of the bilayer using distinct phospholipid compositions. Our results demonstrated that the mitonafide dimer analogue had a comparable encapsulation efficiency (EE%) into the liposomes when loaded into rigid or flexible bilayers in contrast to the low mitonafide monomer EE%. A pH gradient core loading method resulted in a more efficient mechanism to load the monomer into the liposomes. DOSY NMR and spectrofluorometric studies revealed key differences in the structure of the vesicles and the arrangement of the monomer or the dimer in the bilayer or the core of the liposomes. The in vitro assessment of the formulations using MDA-MB-231 and RT-112 cells revealed that a flexible lipid bilayer allows a faster drug release, which correlated well with the spectroscopy studies. This study investigated for the first time that the characteristics of the lipid bilayer and the loading method influence the encapsulation efficacy, colloidal properties, photoactivity and stability of mono and bis-naphthalimides loaded in a liposomal carrier, essential factors that will impact the performance of the formulation in a biological scenario.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Berlanga J. M. C., Brana M. F. and Roldan C. M., Patent, DE2318136A1, 1973
-
- Banerjee S. Veale E. B. Phelan C. M. Murphy S. A. Tocci G. M. Gillespie L. J. Frimannsson D. O. Kelly J. M. Gunnlaugsson T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013;42:1601. doi: 10.1039/C2CS35467E. - DOI - PubMed
-
- Braña M. F. Sanz A. M. Castellano J. M. Roldan C. M. Roldan C. Synthesis and cytostatic activity of benz de isoquinoline 1 3 diones structure activity relationships. Eur. J. Med. Chem. 1981;16:207–212.
-
- U.S. National, Library of Medicine, Clinicaltrials.gov, https://clinicaltrials.gov/ct2/home
LinkOut - more resources
Full Text Sources
Miscellaneous
