Real-World Experience With Avacopan in Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis
- PMID: 38899183
- PMCID: PMC11184253
- DOI: 10.1016/j.ekir.2024.03.022
Real-World Experience With Avacopan in Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis
Abstract
Introduction: Postmarketing data on outcomes of avacopan use in antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) are lacking.
Methods: We performed a multicenter retrospective analysis of 92 patients with newly diagnosed or relapsing AAV who received therapy with avacopan. The coprimary outcome measures were clinical remission at 26 and 52 weeks. We use descriptive statistics and univariate logistic regression to assess outcomes and predictors of remission, respectively.
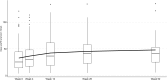
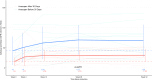
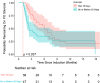
Results: Of the 92 patients, 23% (n = 21) had a baseline estimated glomerular filtration rate (eGFR) < 15 ml/min per 1.73 m2 and 10% on kidney replacement therapy at baseline. Among those with kidney involvement, mean (SD) enrollment eGFR was 33 (27) ml/min per 1.73 m2 with a mean (SD) change of +12 (25) and +20 (23) ml/min per 1.73 m2 at weeks 26 and 52, respectively. In addition to avacopan, 47% of patients received combination therapy of rituximab and low-dose cyclophosphamide, and 14% of patients received plasma exchange (PLEX). After induction, the median (interquartile range [IQR]) time to start avacopan was 3.6 (2.1-7.7) weeks, and the median time to discontinue prednisone after starting avacopan was 5.6 (3.3-9.5) weeks. Clinical remission was achieved in 90% of patients at week 26 and 84% of patients at week 52. Of the patients, 20% stopped avacopan due to adverse events, with the most common being elevated serum aminotransferases (4.3%).
Conclusion: A high rate of remission and an acceptable safety profile were observed with the use of avacopan in the treatment of AAV in this postmarketing analysis, including the populations excluded from the ADVOCATE trial.
Keywords: ANCA-associated vasculitis; avacopan; complement; kidney recovery; remission.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

