Fibroblast Smad7 Induction Protects the Remodeling Pressure-Overloaded Heart
- PMID: 38899461
- PMCID: PMC11257802
- DOI: 10.1161/CIRCRESAHA.123.323360
Fibroblast Smad7 Induction Protects the Remodeling Pressure-Overloaded Heart
Erratum in
-
Correction to: Fibroblast Smad7 Induction Protects the Remodeling Pressure-Overloaded Heart.Circ Res. 2025 Jun 6;136(12):e129. doi: 10.1161/RES.0000000000000716. Epub 2025 Jun 5. Circ Res. 2025. PMID: 40472059 No abstract available.
Abstract
Background: Cardiac fibroblast activation contributes to adverse remodeling, fibrosis, and dysfunction in the pressure-overloaded heart. Although early fibroblast TGF-β (transforming growth factor-β)/Smad (small mother against decapentaplegic)-3 activation protects the pressure-overloaded heart by preserving the matrix, sustained TGF-β activation is deleterious, accentuating fibrosis and dysfunction. Thus, endogenous mechanisms that negatively regulate the TGF-β response in fibroblasts may be required to protect from progressive fibrosis and adverse remodeling. We hypothesized that Smad7, an inhibitory Smad that restrains TGF-β signaling, may be induced in the pressure-overloaded myocardium and may regulate fibrosis, remodeling, and dysfunction.
Methods: The effects of myofibroblast-specific Smad7 loss were studied in a mouse model of transverse aortic constriction, using echocardiography, histological analysis, and molecular analysis. Proteomic studies in S7KO (Smad7 knockout) and overexpressing cells were used to identify fibroblast-derived mediators modulated by Smad7. In vitro experiments using cultured cardiac fibroblasts, fibroblasts populating collagen lattices, and isolated macrophages were used to dissect the molecular signals responsible for the effects of Smad7.
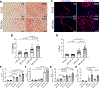
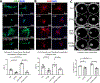
Results: Following pressure overload, Smad7 was upregulated in cardiac myofibroblasts. TGF-β and angiotensin II stimulated fibroblast Smad7 upregulation via Smad3, whereas GDF15 (growth differentiation factor 15) induced Smad7 through GFRAL (glial cell line-derived neurotrophic factor family receptor α-like). MFS7KO (myofibroblast-specific S7KO) mice had increased mortality, accentuated systolic dysfunction and dilative remodeling, and accelerated diastolic dysfunction in response to transverse aortic constriction. Increased dysfunction in MFS7KO hearts was associated with accentuated fibrosis and increased MMP (matrix metalloproteinase)-2 activity and collagen denaturation. Secretomic analysis showed that Smad7 loss accentuates secretion of structural collagens and matricellular proteins and markedly increases MMP2 secretion. In contrast, Smad7 overexpression reduced MMP2 levels. In fibroblasts populating collagen lattices, the effects of Smad7 on fibroblast-induced collagen denaturation and pad contraction were partly mediated via MMP2 downregulation. Surprisingly, MFS7KO mice also exhibited significant macrophage expansion caused by paracrine actions of Smad7 null fibroblasts that stimulate macrophage proliferation and fibrogenic activation. Macrophage activation involved the combined effects of the fibroblast-derived matricellular proteins CD5L (CD5 antigen-like), SPARC (secreted protein acidic and rich in cysteine), CTGF (connective tissue growth factor), ECM1 (extracellular matrix protein 1), and TGFBI (TGFB induced).
Conclusions: The antifibrotic effects of Smad7 in the pressure-overloaded heart protect from dysfunction and involve not only reduction in collagen deposition but also suppression of MMP2-mediated matrix denaturation and paracrine effects that suppress macrophage activation through inhibition of matricellular proteins.
Keywords: collagen; echocardiography; fibroblasts; fibrosis; growth factors; heart failure; macrophages.
Conflict of interest statement
None.
Figures






References
-
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014; 115:625–635. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

