Senescence induces miR-409 to down-regulate CCL5 and impairs angiogenesis in endothelial progenitor cells
- PMID: 38899522
- PMCID: PMC11187746
- DOI: 10.1111/jcmm.18489
Senescence induces miR-409 to down-regulate CCL5 and impairs angiogenesis in endothelial progenitor cells
Abstract
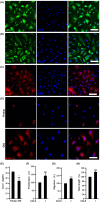
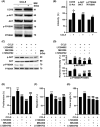
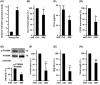
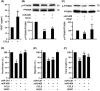
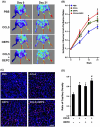
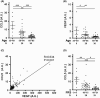
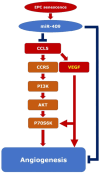
This study explores the impact of senescence on autocrine C-C motif chemokine ligand 5 (CCL5) in human endothelial progenitor cell (EPCs), addressing the poorly understood decline in number and function of EPCs during ageing. We examined the effects of replication-induced senescence on CCL5/CCL5 receptor (CCR5) signalling and angiogenic activity of EPCs in vitro and in vivo. We also explored microRNAs controlling CCL5 secretion in senescent EPCs, its impact on EPC angiogenic activity, and validated our findings in humans. CCL5 secretion and CCR5 levels in senescent EPCs were reduced, leading to attenuated angiogenic activity. CCL5 enhanced EPC proliferation via the CCR5/AKT/P70S6K axis and increased vascular endothelial growth factor (VEGF) secretion. Up-regulation of miR-409 in senescent EPCs resulted in decreased CCL5 secretion, inhibiting the angiogenic activity, though these negative effects were counteracted by the addition of CCL5 and VEGF. In a mouse hind limb ischemia model, CCL5 improved the angiogenic activity of senescent EPCs. Analysis involving 62 healthy donors revealed a negative association between CCL5 levels, age and Framingham Risk Score. These findings propose CCL5 as a potential biomarker for detection of EPC senescence and cardiovascular risk assessment, suggesting its therapeutic potential for age-related cardiovascular disorders.
Keywords: CCL5; angiogenesis; endothelial progenitor cells; miR‐409; senescence.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
CCL5 promotes VEGF-dependent angiogenesis by down-regulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells.Oncotarget. 2014 Nov 15;5(21):10718-31. doi: 10.18632/oncotarget.2532. Oncotarget. 2014. PMID: 25301739 Free PMC article.
-
Hsa-miR-134-5p predicts cardiovascular risk in circulating mononuclear cells and improves angiogenic action of senescent endothelial progenitor cells.J Cell Mol Med. 2024 Jul;28(13):e18523. doi: 10.1111/jcmm.18523. J Cell Mol Med. 2024. PMID: 38957039 Free PMC article.
-
CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment.Carcinogenesis. 2015 Jan;36(1):104-14. doi: 10.1093/carcin/bgu218. Epub 2014 Oct 20. Carcinogenesis. 2015. PMID: 25330803
-
Endothelial progenitor cells and chronic obstructive pulmonary disease: From basic research to clinical application.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Dec 28;49(12):1966-1972. doi: 10.11817/j.issn.1672-7347.2024.240412. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 40195669 Free PMC article. Review. Chinese, English.
-
Endothelial Progenitors: A Consensus Statement on Nomenclature.Stem Cells Transl Med. 2017 May;6(5):1316-1320. doi: 10.1002/sctm.16-0360. Epub 2017 Mar 10. Stem Cells Transl Med. 2017. PMID: 28296182 Free PMC article. Review.
Cited by
-
Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice.Clin Transl Med. 2025 Apr;15(4):e70313. doi: 10.1002/ctm2.70313. Clin Transl Med. 2025. PMID: 40268524 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- RD-110-1B-P013/MacKay Medical College
- 1091B15/MacKay Medical College
- 107-2632-B-715-001/Institute for Information Industry, Ministry of Science and Technology, Taiwan
- 106-2632-B-715-001/Institute for Information Industry, Ministry of Science and Technology, Taiwan
- 105-2632-B-715-001/Institute for Information Industry, Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources

