Modelling bone metastasis in spheroids to study cancer progression and screen cisplatin efficacy
- PMID: 38899562
- PMCID: PMC11503253
- DOI: 10.1111/cpr.13693
Modelling bone metastasis in spheroids to study cancer progression and screen cisplatin efficacy
Abstract
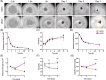
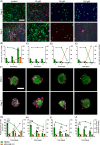
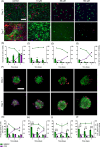
Most bone metastases are caused by primary breast or prostate cancer cells settling in the bone microenvironment, affecting normal bone physiology and function and reducing 5-year survival rates to 10% and 6%, respectively. To expedite clinical availability of novel and effective bone metastases treatments, reliable and predictive in vitro models are urgently required to screen for novel therapies as current in vitro 2D planar mono-culture models do not accurately predict the clinical efficacy. We herein engineered a novel human in vitro 3D co-culture model based on spheroids to study dynamic cellular quantities of (breast or prostate) cancer cells and human bone marrow stromal cells and screen chemotherapeutic efficacy and specificity of the common anticancer drug cisplatin. Bone metastatic spheroids (BMSs) were formed rapidly within 24 h, while the morphology of breast versus prostate cancer BMS differed in terms of size and circularity upon prolonged culture periods. Prestaining cell types prior to BMS formation enabled confocal imaging and quantitative image analysis of in-spheroid cellular dynamics for up to 7 days of BMS culture. We found that cancer cells in BMS proliferated faster and were less susceptible to cisplatin treatment compared to 2D control cultures. Based on these findings and the versatility of our methodology, BMS represent a feasible 3D in vitro model for screening of new bone cancer metastases therapies.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures



Similar articles
-
In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening.Acta Biomater. 2019 Aug;94:392-409. doi: 10.1016/j.actbio.2019.06.012. Epub 2019 Jun 12. Acta Biomater. 2019. PMID: 31200118
-
Using high throughput microtissue culture to study the difference in prostate cancer cell behavior and drug response in 2D and 3D co-cultures.BMC Cancer. 2018 May 24;18(1):592. doi: 10.1186/s12885-018-4473-8. BMC Cancer. 2018. PMID: 29793440 Free PMC article.
-
Mesenchymal Stem Cells Increase Drug Tolerance of A431 Cells Only in 3D Spheroids, Not in 2D Co-Cultures.Int J Mol Sci. 2024 Apr 20;25(8):4515. doi: 10.3390/ijms25084515. Int J Mol Sci. 2024. PMID: 38674102 Free PMC article.
-
Three-Dimensional Cell Cultures as an In Vitro Tool for Prostate Cancer Modeling and Drug Discovery.Int J Mol Sci. 2020 Sep 16;21(18):6806. doi: 10.3390/ijms21186806. Int J Mol Sci. 2020. PMID: 32948069 Free PMC article. Review.
-
Tissue-engineered 3D models for elucidating primary and metastatic bone cancer progression.Acta Biomater. 2019 Nov;99:18-32. doi: 10.1016/j.actbio.2019.08.020. Epub 2019 Aug 13. Acta Biomater. 2019. PMID: 31419564 Free PMC article. Review.
Cited by
-
Nano-Drug Delivery Systems for Bone Metastases: Targeting the Tumor-Bone Microenvironment.Pharmaceutics. 2025 May 2;17(5):603. doi: 10.3390/pharmaceutics17050603. Pharmaceutics. 2025. PMID: 40430894 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

