Preferential crystallization of (±)-pinenyllithium·TMEDA
- PMID: 38899749
- PMCID: PMC11225615
- DOI: 10.1107/S2053229624004662
Preferential crystallization of (±)-pinenyllithium·TMEDA
Abstract
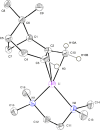
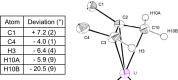
(±)-Pinenyllithium·TMEDA or (tetramethylethylenediamine-κ2N,N')(η3-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptyl)lithium, [Li(C10H15)(C6H16N2)], is readily prepared from β-pinene, butyllithium and TMEDA, and the racemic material preferentially crystallizes even from 96:4 (92% ee) mixtures of (-)- and (+)-β-pinene, respectively. The structure is monomeric, with the geminal-dimethyl bridge of the bicyclic structure shielding one face of the allyl system, restricting the lithium to the opposite face and preventing the Li-allyl-Li aggregation observed with some other allyllithium systems. The symmetry of the allyl system, bond lengths, bond angles and out-of-plane deviations are compared to existing structures. In addition, a much older structure of this complex is compared to this very recent one.
Keywords: TMEDA; allylic metalation; allyllithium; crystal structure; pinene.
Figures

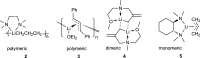
References
-
- Bauer, W., Winchester, W. & Schleyer, P. R. (1987). Organometallics, 6, 2371–2379.
-
- Begley, M. J., Gill, G. B. & Wallace, B. (1978). J. Chem. Soc. Perkin Trans. 1, pp. 93–97.
-
- Boche, G., Etzrodt, H., Marsch, M., Massa, W., Baum, G., Dietrich, H. & Mahdi, W. (1986). Angew. Chem. Int. Ed. Engl.25, 104–105.
-
- Boche, G., Fraenkel, G., Cabral, J., Harms, K., Van Eikema Hommes, N. J. R., Lohrenz, J., Marsch, M., Schleyer, P. & v, R. (1992). J. Am. Chem. Soc.114, 1562–1565.
-
- Brown, C. A. (1978). Synthesis (Stuttg), pp. 754–755.
Grants and funding
LinkOut - more resources
Full Text Sources

