A BSL-2 compliant mouse model of SARS-CoV-2 infection for efficient and convenient antiviral evaluation
- PMID: 38899934
- PMCID: PMC11265351
- DOI: 10.1128/jvi.00504-24
A BSL-2 compliant mouse model of SARS-CoV-2 infection for efficient and convenient antiviral evaluation
Abstract
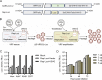
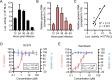
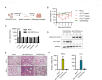
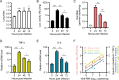
Animal models of authentic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection require operation in biosafety level 3 (BSL-3) containment. In the present study, we established a mouse model employing a single-cycle infectious virus replicon particle (VRP) system of SARS-CoV-2 that can be safely handled in BSL-2 laboratories. The VRP [ΔS-VRP(G)-Luc] contains a SARS-CoV-2 genome in which the spike gene was replaced by a firefly luciferase (Fluc) reporter gene (Rep-Luci), and incorporates the vesicular stomatitis virus glycoprotein on the surface. Intranasal inoculation of ΔS-VRP(G)-Luc can successfully transduce the Rep-Luci genome into mouse lungs, initiating self-replication of Rep-Luci and, accordingly, inducing acute lung injury mimicking the authentic SARS-CoV-2 pathology. In addition, the reporter Fluc expression can be monitored using a bioluminescence imaging approach, allowing a rapid and convenient determination of viral replication in ΔS-VRP(G)-Luc-infected mouse lungs. Upon treatment with an approved anti-SARS-CoV-2 drug, VV116, the viral replication in infected mouse lungs was significantly reduced, suggesting that the animal model is feasible for antiviral evaluation. In summary, we have developed a BSL-2-compliant mouse model of SARS-CoV-2 infection, providing an advanced approach to study aspects of the viral pathogenesis, viral-host interactions, as well as the efficacy of antiviral therapeutics in the future.IMPORTANCESevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is highly contagious and pathogenic in humans; thus, research on authentic SARS-CoV-2 has been restricted to biosafety level 3 (BSL-3) laboratories. However, due to the scarcity of BSL-3 facilities and trained personnel, the participation of a broad scientific community in SARS-CoV-2 research had been greatly limited, hindering the advancement of our understanding on the basic virology as well as the urgently necessitated drug development. Previously, our colleagues Jin et al. had generated a SARS-CoV-2 replicon by replacing the essential spike gene in the viral genome with a Fluc reporter (Rep-Luci), which can be safely operated under BSL-2 conditions. By incorporating the Rep-Luci into viral replicon particles carrying vesicular stomatitis virus glycoprotein on their surface, and via intranasal inoculation, we successfully transduced the Rep-Luci into mouse lungs, developing a mouse model mimicking SARS-CoV-2 infection. Our model can serve as a useful platform for SARS-CoV-2 pathological studies and antiviral evaluation under BSL2 containment.
Keywords: SARS-CoV-2; animal model; replicon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

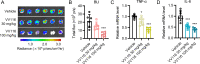
Similar articles
-
Development of a Single-Cycle Infectious SARS-CoV-2 Virus Replicon Particle System for Use in Biosafety Level 2 Laboratories.J Virol. 2022 Feb 9;96(3):e0183721. doi: 10.1128/JVI.01837-21. Epub 2021 Dec 1. J Virol. 2022. PMID: 34851142 Free PMC article.
-
A Replication-Competent Vesicular Stomatitis Virus for Studies of SARS-CoV-2 Spike-Mediated Cell Entry and Its Inhibition.Cell Host Microbe. 2020 Sep 9;28(3):486-496.e6. doi: 10.1016/j.chom.2020.06.020. Epub 2020 Jul 3. Cell Host Microbe. 2020. PMID: 32738193 Free PMC article.
-
SARS-CoV-2 replicon for high-throughput antiviral screening.J Gen Virol. 2021 May;102(5):001583. doi: 10.1099/jgv.0.001583. J Gen Virol. 2021. PMID: 33956592 Free PMC article.
-
SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy.Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020. Theranostics. 2020. PMID: 32642005 Free PMC article. Review.
-
Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past.Front Immunol. 2020 Aug 7;11:1949. doi: 10.3389/fimmu.2020.01949. eCollection 2020. Front Immunol. 2020. PMID: 32849654 Free PMC article. Review.
Cited by
-
Scutellaria barbata D. Don extracts alleviate SARS-CoV-2 induced acute lung injury by inhibiting virus replication and bi-directional immune modulation.Virol Sin. 2025 Jun;40(3):430-438. doi: 10.1016/j.virs.2025.04.004. Epub 2025 Apr 12. Virol Sin. 2025. PMID: 40228743 Free PMC article.
References
-
- Andrade AC dos SP, Campolina-Silva GH, Queiroz-Junior CM, de Oliveira LC, Lacerda L de SB, Pimenta JC, de Souza FRO, de Meira Chaves I, Passos IB, Teixeira DC, et al. . 2021. A biosafety level 2 mouse model for studying betacoronavirus-induced acute lung damage and systemic manifestations. J Virol 95:e0127621. doi:10.1128/jvi.01276-21 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

