Gut mycobiome alterations in obesity in geographically different regions
- PMID: 38899956
- PMCID: PMC11195487
- DOI: 10.1080/19490976.2024.2367297
Gut mycobiome alterations in obesity in geographically different regions
Abstract
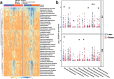
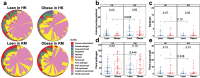
The gut fungi play important roles in human health and are involved in energy metabolism. This study aimed to examine gut mycobiome composition in obese subjects in two geographically different regions in China and to identify specific gut fungi associated with obesity. A total of 217 subjects from two regions with different urbanization levels [Hong Kong (HK): obese, n = 59; lean, n = 59; Kunming (KM): obese, n = 50; lean, n = 49. Mean body mass index (BMI) for obesity = 33.7] were recruited. We performed deep shotgun metagenomic sequencing on fecal samples to compare gut mycobiome composition and trophic functions in lean and obese subjects across these two regions. The gut mycobiome of obese subjects in both HK and KM were altered compared to those of lean subjects, characterized by a decrease in the relative abundance of Nakaseomyces, Schizosaccharomyces pombe, Candida dubliniensis and an increase in the abundance of Lanchanceathermotolerans, Saccharomyces paradox, Parastagonospora nodorum and Myceliophthorathermophila. Reduced fungal - bacterial and fungal - fungal correlations as well as increased negative fungal-bacterial correlations were observed in the gut of obese subjects. Furthermore, the anti-obesity effect of fungus S. pombe was further validated using a mouse model. Supplementing high-fat diet-induced obese mice with the fungus for 12 weeks led to a significant reduction in body weight gain (p < 0.001), and an improvement in lipid and glucose metabolism compared to mice without intervention. In conclusion, the gut mycobiome composition and functionalities of obese subjects were altered. These data shed light on the potential of utilizing fungus-based therapeutics for the treatment of obesity. S. pombe may serve as a potential fungal probiotic in the prevention of diet-induced obesity and future human trials are needed.
Keywords: Obesity; Schizosaccharomyces pombe; dietary habit; fungi dysbiosis; mycobiome.
Conflict of interest statement
FKLC is Board Member of CUHK Medical Centre. He is a co-founder, non-executive Board Chairman, non-executive scientific advisor, honorary Chief Medical Officer and shareholder of GenieBiome Ltd. He receives patent royalties through his affiliated institutions. He has received fees as an advisor and honoraria as a speaker for Eisai Co. Ltd., AstraZeneca, Pfizer Inc., Takeda Pharmaceutical Co., and Takeda (China) Holdings Co. Ltd.
SCN has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie and received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. SCN has received research grants through her affiliated institutions from Olympus, Ferring, and AbbVie. SCN is a founder member, non-executive director, non-executive scientific advisor, and shareholder of GenieBiome Ltd. SCN receives patent royalties through her affiliated institutions.
HZ, YW, ZX, FZ, WZ, WT, YKY, FKLC, TZ and SCN are named inventors of patent applications held by the CUHK and MagIC that cover the therapeutic and diagnostic use of microbiome.
Figures




References
-
- Hales CM, Carroll MD, Fryar CD. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS data brief. 2020;360:1–8. - PubMed
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
