Epidemiology and Management of Pediatric Group A Streptococcal Pneumonia With Parapneumonic Effusion: An Observational Study
- PMID: 38900036
- PMCID: PMC11319078
- DOI: 10.1097/INF.0000000000004418
Epidemiology and Management of Pediatric Group A Streptococcal Pneumonia With Parapneumonic Effusion: An Observational Study
Abstract
Background: During autumn/winter 2022, UK pediatricians reported an unseasonal increase in invasive group A streptococcal infections; a striking proportion presenting with pneumonia with parapneumonic effusion.
Methods: Clinicians across the United Kingdom were requested to submit pseudonymized clinical data using a standardized report form for children (<16 years) admitted between September 30, 2022 and February 17, 2023, with microbiologically confirmed group A streptococcal pneumonia with parapneumonic effusion.
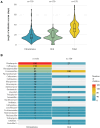
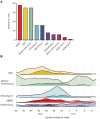
Results: From 185 cases submitted, the median patient age was 4.4 years, and 163 (88.1%) were previously healthy. Respiratory viral coinfection was detected on admission for 101/153 (66.0%) children using extended respiratory pathogen polymerase chain reaction panel. Molecular testing was the primary method of detecting group A streptococcus on pleural fluid (86/171; 50.3% samples). Primary surgical management was undertaken in 171 (92.4%) children; 153/171 (89.4%) had pleural drain inserted (96 with fibrinolytic agent), 14/171 (8.2%) had video-assisted thoracoscopic surgery. Fever duration after admission was prolonged (median, 12 days; interquartile range, 9-16). Intravenous antibiotic courses varied in length (median, 14 days; interquartile range, 12-21), with many children receiving multiple broad-spectrum antibiotics, although evidence for additional bacterial infection was limited.
Conclusions: Most cases occurred with viral coinfection, a previously well-recognized risk with influenza and varicella zoster, highlighting the need to ensure routine vaccination coverage and progress on vaccines for other common viruses (eg, respiratory syncytial virus, human metapneumovirus) and for group A streptococcus. Molecular testing is valuable to detect viral coinfection and confirm invasive group A streptococcal diagnosis, expediting the incorporation of cases into national reporting systems. Range and duration of intravenous antibiotics administered demonstrated the need for research on the optimal duration of antimicrobials and improved stewardship.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Lees EA, Carrol ED. Treating invasive group A Streptococcal infections. Paediatr Child Health. 2014;24:242–247.
-
- Carapetis JR, Steer AC, Mulholland EK, et al. . The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. - PubMed
-
- Cordery R, Purba AK, Begum L, et al. . Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: a prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe. 2022;3:e366–e375. - PMC - PubMed
-
- Bamford A, Whittaker E. Resurgence of group A streptococcal disease in children. BMJ. 2023;380:43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

