CD28 Costimulation Augments CAR Signaling in NK Cells via the LCK/CD3ζ/ZAP70 Signaling Axis
- PMID: 38900051
- PMCID: PMC11452288
- DOI: 10.1158/2159-8290.CD-24-0096
CD28 Costimulation Augments CAR Signaling in NK Cells via the LCK/CD3ζ/ZAP70 Signaling Axis
Abstract
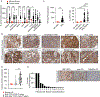
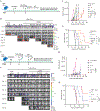
Multiple factors in the design of a chimeric antigen receptor (CAR) influence CAR T-cell activity, with costimulatory signals being a key component. Yet, the impact of costimulatory domains on the downstream signaling and subsequent functionality of CAR-engineered natural killer (NK) cells remains largely unexplored. Here, we evaluated the impact of various costimulatory domains on CAR-NK cell activity, using a CD70-targeting CAR. We found that CD28, a costimulatory molecule not inherently present in mature NK cells, significantly enhanced the antitumor efficacy and long-term cytotoxicity of CAR-NK cells both in vitro and in multiple xenograft models of hematologic and solid tumors. Mechanistically, we showed that CD28 linked to CD3ζ creates a platform that recruits critical kinases, such as lymphocyte-specific protein tyrosine kinase (LCK) and zeta-chain-associated protein kinase 70 (ZAP70), initiating a signaling cascade that enhances CAR-NK cell function. Our study provides insights into how CD28 costimulation enhances CAR-NK cell function and supports its incorporation in NK-based CARs for cancer immunotherapy. Significance: We demonstrated that incorporation of the T-cell-centric costimulatory molecule CD28, which is normally absent in mature natural killer (NK) cells, into the chimeric antigen receptor (CAR) construct recruits key kinases including lymphocyte-specific protein tyrosine kinase and zeta-chain-associated protein kinase 70 and results in enhanced CAR-NK cell persistence and sustained antitumor cytotoxicity.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;380:45–56. - PubMed
-
- Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4–1BB costimulatory domains. Nat Rev Clin Oncol. 2021;18:715–27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

