F18-Choline PET/CT or MIBI SPECT/CT in the Surgical Management of Primary Hyperparathyroidism: A Diagnostic Randomized Clinical Trial
- PMID: 38900416
- PMCID: PMC11190825
- DOI: 10.1001/jamaoto.2024.1421
F18-Choline PET/CT or MIBI SPECT/CT in the Surgical Management of Primary Hyperparathyroidism: A Diagnostic Randomized Clinical Trial
Abstract
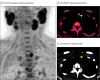
Importance: Whether F18-choline (FCH) positron emission tomographic (PET)/computed tomographic (CT) scan can replace Tc99m-sestaMIBI (MIBI) single-photon emission (SPE)CT/CT as a first-line imaging technique for preoperative localization of parathyroid adenomas (PTA) in patients with primary hyperparathyroidism (PHPT) is unclear.
Objective: To compare first-line FCH PET/CT vs MIBI SPECT/CT for optimal care in patients with PHPT needing parathyroidectomy and to compare the proportions of patients in whom the first-line imaging method resulted in successful minimally invasive parathyroidectomy (MIP) and normalization of calcemia 1 month after surgery.
Design, setting, and participants: A French multicenter randomized open diagnostic intervention phase 3 trial was conducted. Patients were enrolled from November 2019 to May 2022 and participated up to 6 months after surgery. The study included adults with PHPT and an indication for surgical treatment. Patients with previous parathyroid surgery or multiple endocrine neoplasia type 1 (MEN1) were ineligible.
Interventions: Patients were assigned in a 1:1 ratio to receive first-line FCH PET/CT (FCH1) or MIBI SPECT/CT (MIBI1). In the event of negative or inconclusive first-line imaging, they received second-line FCH PET/CT (FCH2) after MIBI1 or MIBI SPECT/CT (MIBI2) after FCH1. All patients underwent surgery under general anesthesia within 12 weeks following the last imaging. Clinical and biologic (serum calcemia and parathyroid hormone levels) assessments were performed 1 and 6 months after surgery.
Main outcomes and measures: The primary outcome was a true-positive first-line imaging-guided MIP combined with uncorrected serum calcium levels of 2.55 mmol/l or less 1 month after surgery, corresponding to the local upper limit of normality.
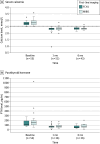
Results: Overall, 57 patients received FCH1 (n = 29) or MIBI1 (n = 28). The mean (SD) age of patients was 62.8 (12.5) years with 15 male (26%) and 42 female (74%) patients. Baseline patient characteristics were similar between groups. Normocalcemia at 1 month after positive first-line imaging-guided MIP was observed in 23 of 27 patients (85%) in the FCH1 group and 14 of 25 patients (56%) in the MIBI1 group. Sensitivity was 82% (95% CI, 62%-93%) and 63% (95% CI, 42%-80%) for FCH1 and MIBI1, respectively. Follow-up at 6 months with biochemical measures was available in 43 patients, confirming that all patients with normocalcemia at 1 month after surgery still had it at 6 months. No adverse events related to imaging and 4 adverse events related to surgery were reported.
Conclusions: This randomized clinical trial found that first-line FCH PET/CT is a suitable and safe replacement for MIBI SPECT/CT. FCH PET/CT leads more patients with PHPT to correct imaging-guided MIP and normocalcemia than MIBI SPECT/CT thanks to its superior sensitivity.
Trial registration: ClinicalTrials.gov Identifier: NCT04040946.
Conflict of interest statement
Figures


References
-
- Beheshti M, Hehenwarter L, Paymani Z, et al. . 18F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: a prospective dual-centre study in 100 patients. Eur J Nucl Med Mol Imaging. 2018;45(10):1762-1771. doi:10.1007/s00259-018-3980-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

