Survival in Patients With Recurrent Intermediate-Stage Hepatocellular Carcinoma: Sorafenib Plus TACE vs TACE Alone Randomized Clinical Trial
- PMID: 38900435
- PMCID: PMC11190833
- DOI: 10.1001/jamaoncol.2024.1831
Survival in Patients With Recurrent Intermediate-Stage Hepatocellular Carcinoma: Sorafenib Plus TACE vs TACE Alone Randomized Clinical Trial
Abstract
Importance: Transarterial chemoembolization (TACE) is commonly used to treat patients with recurrent intermediate-stage hepatocellular carcinoma (HCC) and positive microvascular invasion (MVI); however, TACE alone has demonstrated unsatisfactory survival benefits. A previous retrospective study suggested that TACE plus sorafenib (SOR-TACE) may be a better therapeutic option compared with TACE alone.
Objective: To investigate the clinical outcomes of SOR-TACE vs TACE alone for patients with recurrent intermediate-stage HCC after R0 hepatectomy with positive MVI.
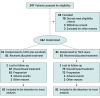
Design, setting, and participants: In this phase 3, open-label, multicenter randomized clinical trial, patients with recurrent intermediate-stage HCC and positive MVI were randomly assigned in a 1:1 ratio via a computerized minimization technique to either SOR-TACE treatment or TACE alone. This trial was conducted at 5 hospitals in China, and enrolled patients from October 2019 to December 2021, with a follow-up period of 24 months. Data were analyzed from June 2023 to September 2023.
Interventions: Randomization to on-demand TACE (conventional TACE: doxorubicin, 50 mg, mixed with lipiodol and gelatin sponge particles [diameter: 150-350 μm]; drug-eluting bead TACE: doxorubicin, 75 mg, mixed with drug-eluting particles [diameter: 100-300 μm or 300-500 μm]) (TACE group) or sorafenib, 400 mg, twice daily plus on-demand TACE (SOR-TACE group) (conventional TACE: doxorubicin, 50 mg, mixed with lipiodol and gelatin sponge particles [diameter, 150-350 μm]; drug-eluting bead TACE: doxorubicin, 75 mg, mixed with drug-eluting particles [diameter: 100-300 μm or 300-500 μm]).
Main outcomes and measures: The primary end point was overall survival by intention-to-treat analysis. Safety was assessed in patients who received at least 1 dose of study treatment.
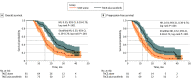
Results: A total of 162 patients (median [range] age, 55 [28-75] years; 151 males [93.2%]), were randomly assigned to be treated with either SOR-TACE (n = 81) or TACE alone (n = 81). The median overall survival was significantly longer in the SOR-TACE group than in the TACE group (22.2 months vs 15.1 months; hazard ratio [HR], 0.55; P < .001). SOR-TACE also prolonged progression-free survival (16.2 months vs 11.8 months; HR, 0.54; P < .001), and improved the objective response rate when compared with TACE alone based on the modified Response Evaluation Criteria in Solid Tumors criteria (80.2% vs 58.0%; P = .002). Any grade adverse events were more common in the SOR-TACE group, but all adverse events responded well to treatment. No unexpected adverse events or treatment-related deaths occurred in this study.
Conclusions and relevance: The results of this randomized clinical trial demonstrated that SOR-TACE achieved better clinical outcomes than TACE alone. These findings suggest that combined treatment should be used for patients with recurrent intermediate-stage HCC after R0 hepatectomy with positive MVI.
Trial registration: ClinicalTrials.gov Identifier: NCT04103398.
Conflict of interest statement
Figures
Similar articles
-
Toripalimab plus bevacizumab versus sorafenib as first-line treatment for advanced hepatocellular carcinoma (HEPATORCH): a randomised, open-label, phase 3 trial.Lancet Gastroenterol Hepatol. 2025 Jul;10(7):658-670. doi: 10.1016/S2468-1253(25)00059-7. Epub 2025 May 20. Lancet Gastroenterol Hepatol. 2025. PMID: 40409323 Clinical Trial.
-
Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): a multiregional, randomised, double-blind, placebo-controlled, phase 3 study.Lancet. 2025 Jan 18;405(10474):216-232. doi: 10.1016/S0140-6736(24)02551-0. Epub 2025 Jan 8. Lancet. 2025. PMID: 39798579 Free PMC article. Clinical Trial.
-
Transarterial chemoembolization plus apatinib for unresectable hepatocellular carcinoma: a multicenter, randomized, open-label, phase III trial.BMC Med. 2025 May 28;23(1):313. doi: 10.1186/s12916-025-04159-y. BMC Med. 2025. PMID: 40437469 Free PMC article. Clinical Trial.
-
Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis.BMC Gastroenterol. 2018 Sep 4;18(1):138. doi: 10.1186/s12876-018-0849-0. BMC Gastroenterol. 2018. PMID: 30180810 Free PMC article.
-
Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis.Mol Biol Rep. 2014 Oct;41(10):6575-82. doi: 10.1007/s11033-014-3541-7. Epub 2014 Aug 5. Mol Biol Rep. 2014. PMID: 25091939
Cited by
-
Lasso-Based Nomogram for Predicting Early Recurrence Following Radical Resection in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2025 Mar 12;12:539-552. doi: 10.2147/JHC.S510581. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40099228 Free PMC article.
-
Correlation of radiotherapy, targeted therapy, and immunotherapy with hepatocellular carcinoma recurrence.World J Gastrointest Oncol. 2025 Jul 15;17(7):107815. doi: 10.4251/wjgo.v17.i7.107815. World J Gastrointest Oncol. 2025. PMID: 40697226 Free PMC article. Review.
-
Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2025 Jun 24;17(13):2110. doi: 10.3390/cancers17132110. Cancers (Basel). 2025. PMID: 40647409 Free PMC article. Review.
-
Sorafenib with or without co-interventions for hepatocellular carcinoma.Cochrane Database Syst Rev. 2025 Jun 26;6(6):CD015851. doi: 10.1002/14651858.CD015851. Cochrane Database Syst Rev. 2025. PMID: 40568833 Free PMC article. Review.
-
Determinants of Health-Related Quality of Life After Transarterial Chemoembolization in Hepatocellular Carcinoma Patients: A Systematic Review.J Clin Med. 2025 Jun 3;14(11):3941. doi: 10.3390/jcm14113941. J Clin Med. 2025. PMID: 40507701 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

