A Clinical Diagnostic Test for Calcium Release Deficiency Syndrome
- PMID: 38900490
- PMCID: PMC11190834
- DOI: 10.1001/jama.2024.8599
A Clinical Diagnostic Test for Calcium Release Deficiency Syndrome
Abstract
Importance: Sudden death and cardiac arrest frequently occur without explanation, even after a thorough clinical evaluation. Calcium release deficiency syndrome (CRDS), a life-threatening genetic arrhythmia syndrome, is undetectable with standard testing and leads to unexplained cardiac arrest.
Objective: To explore the cardiac repolarization response on an electrocardiogram after brief tachycardia and a pause as a clinical diagnostic test for CRDS.
Design, setting, and participants: An international, multicenter, case-control study including individual cases of CRDS, 3 patient control groups (individuals with suspected supraventricular tachycardia; survivors of unexplained cardiac arrest [UCA]; and individuals with genotype-positive catecholaminergic polymorphic ventricular tachycardia [CPVT]), and genetic mouse models (CRDS, wild type, and CPVT were used to define the cellular mechanism) conducted at 10 centers in 7 countries. Patient tracings were recorded between June 2005 and December 2023, and the analyses were performed from April 2023 to December 2023.
Intervention: Brief tachycardia and a subsequent pause (either spontaneous or mediated through cardiac pacing).
Main outcomes and measures: Change in QT interval and change in T-wave amplitude (defined as the difference between their absolute values on the postpause sinus beat and the last beat prior to tachycardia).
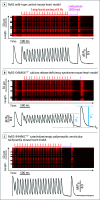
Results: Among 10 case patients with CRDS, 45 control patients with suspected supraventricular tachycardia, 10 control patients who experienced UCA, and 3 control patients with genotype-positive CPVT, the median change in T-wave amplitude on the postpause sinus beat (after brief ventricular tachycardia at ≥150 beats/min) was higher in patients with CRDS (P < .001). The smallest change in T-wave amplitude was 0.250 mV for a CRDS case patient compared with the largest change in T-wave amplitude of 0.160 mV for a control patient, indicating 100% discrimination. Although the median change in QT interval was longer in CRDS cases (P = .002), an overlap between the cases and controls was present. The genetic mouse models recapitulated the findings observed in humans and suggested the repolarization response was secondary to a pathologically large systolic release of calcium from the sarcoplasmic reticulum.
Conclusions and relevance: There is a unique repolarization response on an electrocardiogram after provocation with brief tachycardia and a subsequent pause in CRDS cases and mouse models, which is absent from the controls. If these findings are confirmed in larger studies, this easy to perform maneuver may serve as an effective clinical diagnostic test for CRDS and become an important part of the evaluation of cardiac arrest.
Trial registration: ClinicalTrials.gov NCT06188689.
Conflict of interest statement
Figures



Comment on
-
The First Clinical Test for Calcium Release Deficiency Syndrome?JAMA. 2024 Jul 16;332(3):214. doi: 10.1001/jama.2024.10230. JAMA. 2024. PMID: 38900415 No abstract available.
References
-
- Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

