New Frontiers in Nonheme Enzymatic Oxyferryl Species
- PMID: 38900645
- PMCID: PMC11983317
- DOI: 10.1002/cbic.202400307
New Frontiers in Nonheme Enzymatic Oxyferryl Species
Abstract
Non-heme mononuclear iron dependent (NHM-Fe) enzymes exhibit exceedingly diverse catalytic reactivities. Despite their catalytic versatilities, the mononuclear iron centers in these enzymes show a relatively simple architecture, in which an iron atom is ligated with 2-4 amino acid residues, including histidine, aspartic or glutamic acid. In the past two decades, a common high-valent reactive iron intermediate, the S=2 oxyferryl (Fe(IV)-oxo or Fe(IV)=O) species, has been repeatedly discovered in NHM-Fe enzymes containing a 2-His-Fe or 2-His-1-carboxylate-Fe center. However, for 3-His/4-His-Fe enzymes, no common reactive intermediate has been identified. Recently, we have spectroscopically characterized the first S=1 Fe(IV) intermediate in a 3-His-Fe containing enzyme, OvoA, which catalyzes a novel oxidative carbon-sulfur bond formation. In this review, we summarize the broad reactivities demonstrated by S=2 Fe(IV)-oxo intermediates, the discovery of the first S=1 Fe(IV) intermediate in OvoA and the mechanistic implication of such a discovery, and the intrinsic reactivity differences of the S=2 and the S=1 Fe(IV)-oxo species. Finally, we postulate the possible reasons to utilize an S=1 Fe(IV) species in OvoA and their implications to other 3-His/4-His-Fe enzymes.
Keywords: C−H activation; Ferryl intermediates; Nonheme iron enzymes; Oxidative C−S bond formation; Spin state.
© 2024 The Author(s). ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
Figures
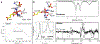

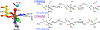
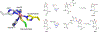



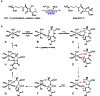
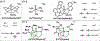
References
-
- Schofield C and Hausinger R, Eds., 2-Oxoglutarate-Dependent Oxygenases, Royal Society of Chemistry, Cambridge, 2015.
-
- Guo Y, Chang W, Li J and Davidson M, in Comprehensive Coordination Chemistry III, eds. Constable EC, Parkin G and Que LBT-CCCIII Jr, Elsevier, Oxford, 2021, pp. 301–332.
-
- Guo Y, Chang W, Li J and Davidson M, in Comprehensive Coordination Chemistry III, eds. Constable EC, Parkin G and Que LBT-CCCIII Jr, Elsevier, Oxford, 2021, pp. 269–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

