Visualization of Keratopathy Associated With the Antibody-Drug Conjugate Belantamab Mafodotin Using Infrared Imaging in Patients With Multiple Myeloma
- PMID: 38900711
- PMCID: PMC11676612
- DOI: 10.1097/ICO.0000000000003596
Visualization of Keratopathy Associated With the Antibody-Drug Conjugate Belantamab Mafodotin Using Infrared Imaging in Patients With Multiple Myeloma
Abstract
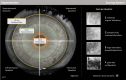
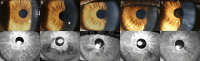
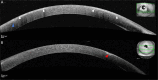
Purpose: The treatment of patients with relapsed/refractory multiple myeloma (RRMM) with the antibody-drug conjugate belantamab mafodotin is affected by ocular adverse effects, most frequently keratopathy with corneal microcyst-like epithelial changes (MECs). To assess ocular side effects, the Keratopathy and Visual Acuity (KVA) scale, based on the extent of keratopathy subjectively graded on slit-lamp examination and the change in best corrected visual acuity from baseline, was created. Advanced corneal imaging techniques have been explored to further characterize MECs and identify objective imaging biomarkers. We examined whether infrared reflectance imaging of the anterior segment (AS-IR) could contribute to the assessment, monitoring, and documentation of corneal toxicity in patients treated with belantamab mafodotin.
Methods: In addition to the KVA examination, AS-IR imaging was performed. AS-IR images were evaluated for presence of visible hyporeflective lesions and their spatial and temporal distribution between visits and compared with keratopathy identified on slit-lamp examination. To standardize the assessment, a scoring system for lesions on AS-IR was implemented for additional analysis.
Results: Nine patients undergoing treatment with belantamab mafodotin for up to 9 months were examined. All patients exhibited hyporeflective lesions on AS-IR imaging, indicative of corneal toxicity corresponding to MECs observed on slit-lamp examination. AS-IR lesions showed early occurrence, variable quantity and size, and distinct distribution patterns, correlating with clinical findings during treatment.
Conclusions: As shown for belantamab mafodotin, AS-IR imaging represents a fast, noninvasive, supplemental method for documentation, monitoring, and assessment of corneal adverse effects during treatment with antibody-drug conjugates, which may enable more standardized analyses.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures



Similar articles
-
Structural changes of corneal epithelium in belantamab-associated superficial keratopathy using anterior segment optical coherence tomography.Am J Ophthalmol Case Rep. 2021 Jun 9;23:101133. doi: 10.1016/j.ajoc.2021.101133. eCollection 2021 Sep. Am J Ophthalmol Case Rep. 2021. PMID: 34169181 Free PMC article.
-
Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM).Blood Cancer J. 2021 May 26;11(5):103. doi: 10.1038/s41408-021-00494-4. Blood Cancer J. 2021. PMID: 34039952 Free PMC article. Clinical Trial.
-
Corneal in vivo confocal microscopy to detect belantamab mafodotin-induced ocular toxicity early and adjust the dose accordingly: a case report.J Hematol Oncol. 2021 Oct 3;14(1):159. doi: 10.1186/s13045-021-01172-5. J Hematol Oncol. 2021. PMID: 34602074 Free PMC article.
-
Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety.Drug Des Devel Ther. 2021 Jun 2;15:2401-2415. doi: 10.2147/DDDT.S267404. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34103900 Free PMC article. Review.
-
Ocular Toxicity of Belantamab Mafodotin, an Oncological Perspective of Management in Relapsed and Refractory Multiple Myeloma.Front Oncol. 2021 May 11;11:678634. doi: 10.3389/fonc.2021.678634. eCollection 2021. Front Oncol. 2021. PMID: 34046363 Free PMC article. Review.
References
-
- Lonial S, Lee HC, Badros A, et al. . Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

