Alpha-defensin binding expands human adenovirus tropism
- PMID: 38900833
- PMCID: PMC11230588
- DOI: 10.1371/journal.ppat.1012317
Alpha-defensin binding expands human adenovirus tropism
Abstract
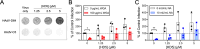
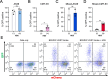
Mammalian α-defensins are a family of abundant effector peptides of the mucosal innate immune system. Although primarily considered to be antimicrobial, α-defensins can increase rather than block infection by certain prominent bacterial and viral pathogens in cell culture and in vivo. We have shown previously that exposure of mouse and human adenoviruses (HAdVs) to α-defensins is able to overcome competitive inhibitors that block cell binding, leading us to hypothesize a defensin-mediated binding mechanism that is independent of known viral receptors. To test this hypothesis, we used genetic approaches to demonstrate that none of several primary receptors nor integrin co-receptors are needed for human α-defensin-mediated binding of HAdV to cells; however, infection remains integrin dependent. Thus, our studies have revealed a novel pathway for HAdV binding to cells that bypasses viral primary receptors. We speculate that this pathway functions in parallel with receptor-mediated entry and contributes to α-defensin-enhanced infection of susceptible cells. Remarkably, we also found that in the presence of α-defensins, HAdV tropism is expanded to non-susceptible cells, even when viruses are exposed to a mixture of both susceptible and non-susceptible cells. Therefore, we propose that in the presence of sufficient concentrations of α-defensins, such as in the lung or gut, integrin expression rather than primary receptor expression will dictate HAdV tropism in vivo. In summary, α-defensins may contribute to tissue tropism not only through the neutralization of susceptible viruses but also by allowing certain defensin-resistant viruses to bind to cells independently of previously described mechanisms.
Copyright: © 2024 Zhao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Update of
-
Alpha-defensin binding expands human adenovirus tropism.bioRxiv [Preprint]. 2024 May 30:2024.05.30.596681. doi: 10.1101/2024.05.30.596681. bioRxiv. 2024. Update in: PLoS Pathog. 2024 Jun 20;20(6):e1012317. doi: 10.1371/journal.ppat.1012317. PMID: 38854108 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

