Embryonic alcohol exposure in zebrafish predisposes adults to cardiomyopathy and diastolic dysfunction
- PMID: 38900908
- PMCID: PMC11535724
- DOI: 10.1093/cvr/cvae139
Embryonic alcohol exposure in zebrafish predisposes adults to cardiomyopathy and diastolic dysfunction
Abstract
Aims: Fetal alcohol spectrum disorders (FASDs) impact up to 0.8% of the global population. However, cardiovascular health outcomes in adult patients, along with predictive biomarkers for cardiac risk stratification, remain unknown. Our aim was to utilize a longitudinal cohort study in an animal model to evaluate the impact of embryonic alcohol exposure (EAE) on cardiac structure, function, and transcriptional profile across the lifespan.
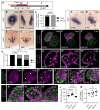
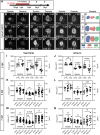
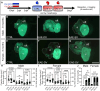
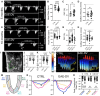
Methods and results: Using zebrafish, we characterized the aftereffects of EAE in adults binned by congenital heart defect (CHD) severity. Chamber sizes were quantified on dissected adult hearts to identify structural changes indicative of cardiomyopathy. Using echocardiography, we quantified systolic function based on ejection fraction and longitudinal strain, and diastolic function based on ventricular filling dynamics, ventricular wall movement, and estimated atrial pressures. Finally, we performed RNA-sequencing on EAE ventricles and assessed how differentially expressed genes (DEGs) correlated with cardiac function. Here, we demonstrate that EAE causes cardiomyopathy and diastolic dysfunction through persistent alterations to ventricular wall structure and gene expression. Following abnormal ventricular morphogenesis, >30% of all EAE adults developed increased atrial-to-ventricular size ratios, abnormal ventricular filling dynamics, and reduced myocardial wall relaxation during early diastole despite preserved systolic function. RNA-sequencing of the EAE ventricle revealed novel and heart failure-associated genes (slc25a33, ankrd9, dusp2, dusp4, spry4, eya4, and edn1) whose expression levels were altered across the animal's lifespan or correlated with the degree of diastolic dysfunction detected in adulthood.
Conclusion: Our study identifies EAE as a risk factor for adult-onset cardiomyopathy and diastolic dysfunction, regardless of CHD status, and suggests novel molecular indicators of adult EAE-induced heart disease.
Keywords: Diastolic dysfunction; Fetal alcohol spectrum disorders; Prenatal alcohol exposure.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures


Similar articles
-
Non-invasive estimation of left ventricular chamber stiffness using cardiovascular magnetic resonance and echocardiography.J Cardiovasc Magn Reson. 2025 Summer;27(1):101849. doi: 10.1016/j.jocmr.2025.101849. Epub 2025 Jan 31. J Cardiovasc Magn Reson. 2025. PMID: 39894254 Free PMC article.
-
Integrating Left Atrial Reservoir Strain Into the First-Line Assessment of Diastolic Function: Prognostic Implications in a Community-Based Cohort With Normal Left Ventricular Systolic Function.J Am Soc Echocardiogr. 2025 Jul;38(7):570-582. doi: 10.1016/j.echo.2025.03.012. Epub 2025 Mar 27. J Am Soc Echocardiogr. 2025. PMID: 40157554
-
Evaluating sex-specific responses to western diet across the lifespan: impact on cardiac function and transcriptomic signatures in C57BL/6J mice at 530 and 640/750 days of age.Cardiovasc Diabetol. 2024 Dec 28;23(1):454. doi: 10.1186/s12933-024-02565-9. Cardiovasc Diabetol. 2024. PMID: 39732652 Free PMC article.
-
Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis.Am J Hypertens. 2014 Feb;27(2):146-56. doi: 10.1093/ajh/hpt215. Epub 2013 Dec 9. Am J Hypertens. 2014. PMID: 24321879
-
A systematic review: effect of angiotensin converting enzyme inhibition on left ventricular volumes and ejection fraction in patients with a myocardial infarction and in patients with left ventricular dysfunction.Eur J Heart Fail. 2007 Feb;9(2):129-35. doi: 10.1016/j.ejheart.2006.05.002. Epub 2006 Jul 7. Eur J Heart Fail. 2007. PMID: 16829187
Cited by
-
Contraction-induced endocardial id2b plays a dual role in regulating myocardial contractility and valve formation.Elife. 2025 Jul 24;13:RP101151. doi: 10.7554/eLife.101151. Elife. 2025. PMID: 40704996 Free PMC article.
References
-
- Jones KL, Smith DW, Hanson JW. The fetal alcohol syndrome: clinical deliniation. Ann Ny Acad Sci 1976;273:130–137. - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM138407/GM/NIGMS NIH HHS/United States
- R35 HL135831/HL/NHLBI NIH HHS/United States
- Collaborative Initiative on Fetal Alcohol Spectrum Disorders
- U01 AA030185/AA/NIAAA NIH HHS/United States
- Boston Children's Hospital Department of Cardiology
- R01 HL148338/HL/NHLBI NIH HHS/United States
- R01HL139806/NH/NIH HHS/United States
- R01 CA278832/CA/NCI NIH HHS/United States
- R01 HL139806/HL/NHLBI NIH HHS/United States
- R01 HL133254/HL/NHLBI NIH HHS/United States
- T32HL007572/Boston Children's Hospital
- T32 HL007572/HL/NHLBI NIH HHS/United States
- U01AA030185/National Institute on Alcoholism and Alcohol Abuse
- R01 GM125632/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

