A mechanistic modelling approach of the host-microbiota interactions to investigate beneficial symbiotic resilience in the human gut
- PMID: 38900957
- PMCID: PMC11285522
- DOI: 10.1098/rsif.2023.0756
A mechanistic modelling approach of the host-microbiota interactions to investigate beneficial symbiotic resilience in the human gut
Abstract
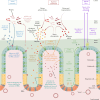
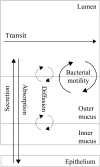
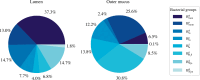
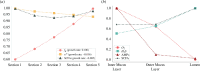
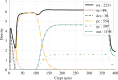
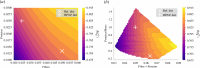
The health and well-being of a host are deeply influenced by the interactions with its gut microbiota. Contrasted environmental conditions, such as diseases or dietary habits, play a pivotal role in modulating these interactions, impacting microbiota composition and functionality. Such conditions can also lead to transitions from beneficial to detrimental symbiosis, viewed as alternative stable states of the host-microbiota dialogue. This article introduces a novel mathematical model exploring host-microbiota interactions, integrating dynamics of the colonic epithelial crypt, microbial metabolic functions, inflammation sensitivity and colon flows in a transverse section. The model considers metabolic shifts in epithelial cells based on butyrate and hydrogen sulfide concentrations, innate immune pattern recognition receptor activation, microbial oxygen tolerance and the impact of antimicrobial peptides on the microbiota. Using the model, we demonstrated that a high-protein, low-fibre diet exacerbates detrimental interactions and compromises beneficial symbiotic resilience, underscoring a destabilizing effect towards an unhealthy state. Moreover, the proposed model provides essential insights into oxygen levels, fibre and protein breakdown, and basic mechanisms of innate immunity in the colon and offers a crucial understanding of factors influencing the colon environment.
Keywords: PDE–ODE coupling; colon flows model; colonic crypt model; host–microbiota interactions modelling; human gut microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
A mathematical model to investigate the key drivers of the biogeography of the colon microbiota.J Theor Biol. 2019 Feb 7;462:552-581. doi: 10.1016/j.jtbi.2018.12.009. Epub 2018 Dec 7. J Theor Biol. 2019. PMID: 30529486
-
Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism.Nutrients. 2020 Oct 6;12(10):3054. doi: 10.3390/nu12103054. Nutrients. 2020. PMID: 33036205 Free PMC article. Review.
-
Reciprocal interactions between gut microbiota and autophagy.World J Gastroenterol. 2021 Dec 28;27(48):8283-8301. doi: 10.3748/wjg.v27.i48.8283. World J Gastroenterol. 2021. PMID: 35068870 Free PMC article. Review.
-
A review of metabolic potential of human gut microbiome in human nutrition.Arch Microbiol. 2018 Mar;200(2):203-217. doi: 10.1007/s00203-017-1459-x. Epub 2017 Nov 29. Arch Microbiol. 2018. PMID: 29188341 Review.
-
Versatile human in vitro triple coculture model coincubated with adhered gut microbes reproducibly mimics pro-inflammatory host-microbe interactions in the colon.FASEB J. 2021 Dec;35(12):e21992. doi: 10.1096/fj.202101135R. FASEB J. 2021. PMID: 34719821
Cited by
-
The role of the microbiota-gut-brain axis and artificial intelligence in cognitive health of pediatric obstructive sleep apnea: A narrative review.Medicine (Baltimore). 2024 Dec 13;103(50):e40900. doi: 10.1097/MD.0000000000040900. Medicine (Baltimore). 2024. PMID: 39686454 Free PMC article. Review.
-
Dietary patterns influencing the human colonic microbiota from infancy to centenarian age: a narrative review.Front Nutr. 2025 Jun 4;12:1591341. doi: 10.3389/fnut.2025.1591341. eCollection 2025. Front Nutr. 2025. PMID: 40535034 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

