Extracellular vesicles from II trimester human amniotic fluid as paracrine conveyors counteracting oxidative stress
- PMID: 38901103
- PMCID: PMC11253147
- DOI: 10.1016/j.redox.2024.103241
Extracellular vesicles from II trimester human amniotic fluid as paracrine conveyors counteracting oxidative stress
Abstract
Background: We previously demonstrated that the human amniotic fluid (hAF) from II trimester of gestation is a feasible source of stromal progenitors (human amniotic fluid stem cells, hAFSC), with significant paracrine potential for regenerative medicine. Extracellular vesicles (EVs) separated and concentrated from hAFSC secretome can deliver pro-survival, proliferative, anti-fibrotic and cardioprotective effects in preclinical models of skeletal and cardiac muscle injury. While hAFSC-EVs isolation can be significantly influenced by in vitro cell culture, here we profiled EVs directly concentrated from hAF as an alternative option and investigated their paracrine potential against oxidative stress.
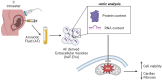
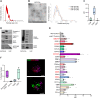
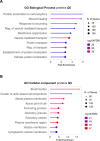
Methods: II trimester hAF samples were obtained as leftover material from prenatal diagnostic amniocentesis following written informed consent. EVs were separated by size exclusion chromatography and concentrated by ultracentrifugation. hAF-EVs were assessed by nanoparticle tracking analysis, transmission electron microscopy, Western Blot, and flow cytometry; their metabolic activity was evaluated by oximetric and luminometric analyses and their cargo profiled by proteomics and RNA sequencing. hAF-EV paracrine potential was tested in preclinical in vitro models of oxidative stress and dysfunction on murine C2C12 cells and on 3D human cardiac microtissue.
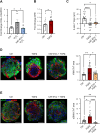
Results: Our protocol resulted in a yield of 6.31 ± 0.98 × 109 EVs particles per hAF milliliter showing round cup-shaped morphology and 209.63 ± 6.10 nm average size, with relevant expression of CD81, CD63 and CD9 tetraspanin markers. hAF-EVs were enriched in CD133/1, CD326, CD24, CD29, and SSEA4 and able to produce ATP by oxygen consumption. While oxidative stress significantly reduced C2C12 survival, hAF-EV priming resulted in significant rescue of cell viability, with notable recovery of ATP synthesis and concomitant reduction of cell damage and lipid peroxidation activity. 3D human cardiac microtissues treated with hAF-EVs and experiencing H2O2 stress and TGFβ stimulation showed improved survival with a remarkable decrease in the onset of fibrosis.
Conclusions: Our results suggest that leftover samples of II trimester human amniotic fluid can represent a feasible source of EVs to counteract oxidative damage on target cells, thus offering a novel candidate therapeutic option to counteract skeletal and cardiac muscle injury.
Keywords: Amniotic fluid; Cell viability; Extracellular vesicles; Metabolic dysfunction; Oxidative stress; Paracrine effect.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have nothing to disclose nor competing interests to declare.
Figures



References
-
- Pozzobon M., D'Agostino S., Roubelakis M.G., Cargnoni A., Gramignoli R., Wolbank S., Gindraux F., Bollini S., Kerdjoudj H., Fenelon M., Di Pietro R., Basile M., Borutinskaite V., Piva R., Schoeberlein A., Eissner G., Giebel B., Ponsaert P. General consensus on multimodal functions and validation analysis of perinatal derivatives for regenerative medicine applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.961987. - DOI - PMC - PubMed
-
- Silini A.R., Di Pietro R., Lang-Olip I., Alviano F., Banerjee A., Basile M., Borutinskaite V., Eissner G., Gellhaus A., Giebel B., Huang Y., Janev A., Erdani Kreft M., Kupper N., Abadia-Molina A.C., Olivares E.G., Pandolfi A., Papait A., Pozzobon M., Ruiz-Ruiz C., Soritau O., Susman S., Szukiewicz D., Weidinger A., Wolbank S., Huppertz B., Parolini O. Perinatal derivatives: where do we stand? A roadmap of the human placenta and consensus for tissue and cell nomenclature. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.610544. - DOI - PMC - PubMed
-
- Papait A., Silini A.R., Gazouli M., Malvicini R., Muraca M., O'Driscoll L., Pacienza N., Toh W.S., Yannarelli G., Ponsaerts P., Parolini O., Eissner G., Pozzobon M., Lim S.K., Giebel B. Perinatal derivatives: how to best validate their immunomodulatory functions. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.981061. - DOI - PMC - PubMed
-
- Bollini S., Cheung K.K., Riegler J., Dong X., Smart N., Ghionzoli M., Loukogeorgakis S.P., Maghsoudlou P., Dube K.N., Riley P.R., Lythgoe M.F., De Coppi P. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cell. Dev. 2011;20:1985–1994. doi: 10.1089/scd.2010.0424. - DOI - PubMed
-
- Zani A., Cananzi M., Fascetti-Leon F., Lauriti G., Smith V.V., Bollini S., Ghionzoli M., D'Arrigo A., Pozzobon M., Piccoli M., Hicks A., Wells J., Siow B., Sebire N.J., Bishop C., Leon A., Atala A., Lythgoe M.F., Pierro A., Eaton S., De Coppi P. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014;63:300–309. doi: 10.1136/gutjnl-2012-303735. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

