DIA-Based Phosphoproteomics Identifies Early Phosphorylation Events in Response to EGTA and Mannitol in Arabidopsis
- PMID: 38901673
- PMCID: PMC11325057
- DOI: 10.1016/j.mcpro.2024.100804
DIA-Based Phosphoproteomics Identifies Early Phosphorylation Events in Response to EGTA and Mannitol in Arabidopsis
Abstract
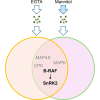
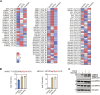
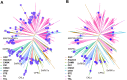
Osmotic stress significantly hampers plant growth and crop yields, emphasizing the need for a thorough comprehension of the underlying molecular responses. Previous research has demonstrated that osmotic stress rapidly induces calcium influx and signaling, along with the activation of a specific subset of protein kinases, notably the Raf-like protein (RAF)-sucrose nonfermenting-1-related protein kinase 2 (SnRK2) kinase cascades within minutes. However, the intricate interplay between calcium signaling and the activation of RAF-SnRK2 kinase cascades remains elusive. Here, in this study, we discovered that Raf-like protein (RAF) kinases undergo hyperphosphorylation in response to osmotic shocks. Intriguingly, treatment with the calcium chelator EGTA robustly activates RAF-SnRK2 cascades, mirroring the effects of osmotic treatment. Utilizing high-throughput data-independent acquisition-based phosphoproteomics, we unveiled the global impact of EGTA on protein phosphorylation. Beyond the activation of RAFs and SnRK2s, EGTA treatment also activates mitogen-activated protein kinase cascades, Calcium-dependent protein kinases, and receptor-like protein kinases, etc. Through overlapping assays, we identified potential roles of mitogen-activated protein kinase kinase kinase kinases and receptor-like protein kinases in the osmotic stress-induced activation of RAF-SnRK2 cascades. Our findings illuminate the regulation of phosphorylation and cellular events by Ca2+ signaling, offering insights into the (exocellular) Ca2+ deprivation during early hyperosmolality sensing and signaling.
Keywords: RAF; SnRK2; calcium signaling; osmotic stress; phosphorylation.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

